IJCRR - 5(17), September, 2013
Pages: 82-89
Date of Publication: 12-Sep-2013
Print Article
Download XML Download PDF
SEASONAL GONADAL BIOCHEMICAL CHANGES, ASSOCIATED WITH THE REPRODUCTIVE CYCLE IN LABEO DYOCHEILUS (MCCLELLAND)
Author: Rakesh Verma
Category: Healthcare
Abstract:Seasonality in reproductive cycles as well as testicular and ovarian biochemical changes is very common among teleost. Seasonal gonadal biochemical changes have potent effect on gonadal maturation and period of spawning. Determination of seasonal variation in percentage value of lipids, protein and water in gonadal tissue and establish the possible correlation with gonadal maturity was the objective of the study. Our study of Gonado-somatic index (GSI) reveals that Monsoon was the spawning season of fish. Gonadal tissue biochemical study showed that the lipid percentage was highest during resting phase of testis and spawning phase of ovary, while lowest during resting phase of ovary and spawning phase of testis. We also found that the monthly variation of lipid concentration in ovary was 1.79 times higher than that of the testis. Protein percentage was highest during spawning phase and lowest during resting phase in both gonads. Monthly variation of protein content in the testis and the ovary was almost equal in percentage. Water percentage was lowest during resting phase of testis and spawning phase of ovary, while highest during resting phase of ovary and spawning phase of testis, water concentration in ovary was 1.10 times higher than testis. Finally Gonado-somatic index correlate with gonadal lipid, protein and water percentage, this study show that the water content (R2 = 0.664) is more responsible for maturation of testis and lipid content (R2 = 0.601) for ovary, as compare to other factors.
Keywords: gonadal lipid, protein, water, gonado-somatic index, reproductive cycle, seasonal changes, Labeo dyocheilus.
Full Text:
INTRODUCTION
It has long been documented that striking changes occur in gonadal biochemical composition in many species of fish during the normal annual sexual cycle. Seasonal reproductive cycles are common among tropical fishes (Robertson, 1990). Earlier investigations illustrate that there are substantial differences in the seasonal gonadal biochemical changes of different species of fishes and among individuals of the same species, with their age, sex and habitat. In general, they have a strong tendency to store the energy particularly, in the form of protein and fat during the active period of spawning. It has now been realized that these changes occur due to various physiological factors such as maturation, spawning and feeding. At any given time, the biochemical composition of an individual fish is the result of complex interactions between physical and biological characteristics such as size, sex, temperature, food availability and reproductive stage. Information of the magnitude of these fluctuations is an indicator of annual fish physiological changes. Various physiological and reproductive changes in fishes also depend upon coordinated actions of various hormones associated with brain-pituitary-gonadal axis (Evans, 1998).
Labeo dyocheilus has been categorized as vulnerable by National Bureau of Fish Genetic Resources (NBFGR) Lucknow, Dubey (1994), Prasad (1994) and CAMP (1998). Objectives of the present work were to determine the percentage value of three main constituents namely: lipids, protein and water in respect to gonadal development and spawning, as well as to establish the possible correlation between gonadal protein, lipids, moisture percentage and reproductive cycle of fresh water fish, Labeo dyocheilus. It is expected that these information will be useful for biologists to investigate various aspect of reproductive biology. This study will fulfill the gap in the literature, as lack of literature in biochemical composition of gonads.
MATERIALS AND METHODS
The sampling was carried out at Chaukhutia (Latitude: 29° 53' 55" N and Longitude: 79° 21' 22" E) in Uttarakhand state of India, annually between September 2009 to August 2012 for the period of three years. About 12 adult individual (25-30 cm in length) in each months were collected. Gonado-somatic index (GSI) is used to indicate gonadal maturation and spawning period. This includes the physical observation of fish and gonads, so as their length-weight was measured. The Gonado-somatic index value was calculated with the help of formula given below:
RESULTS
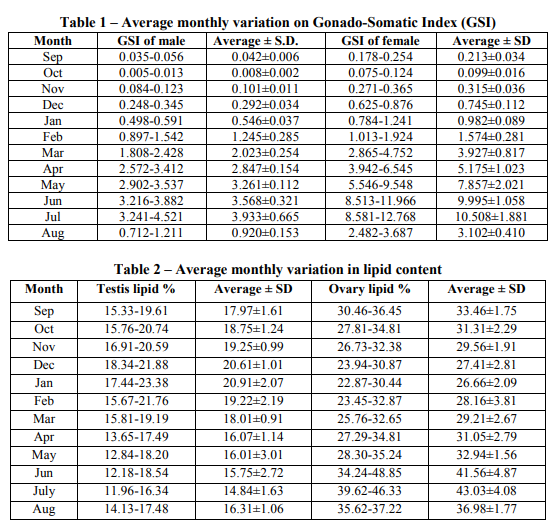
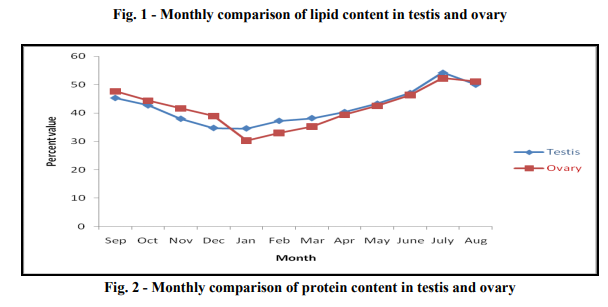
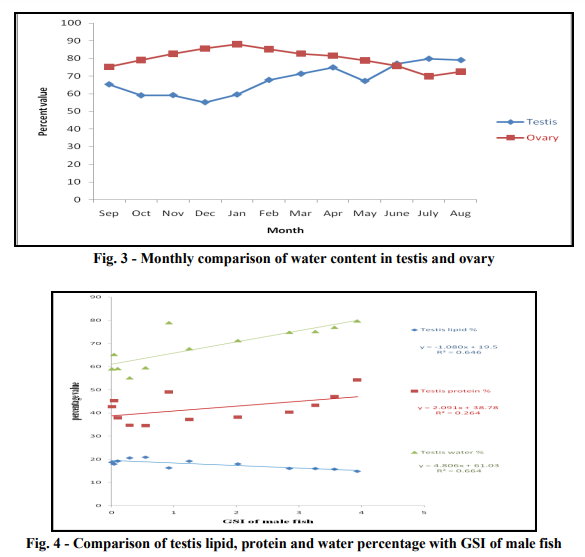
Gonado-somatic index: Minimum value of Gonado-somatic index for male is 0.008 ? 0.002 in October, whereas the maximum value 3.933 ? 0.665 in July. It can also be seen from the Gonadosomatic index data that it increases regularly from the month of October till July and then further decreases. Gonado-somatic index for female was minimum 0.099 ? 0.016 in month of October from which it increases regularly till a maximum value of 10.508 ? 1.881 in month of July. Thus, above result showed the Monsoon (Gonado-somatic index peak value in July) was the spawning season for both male and female fish. (Table1) Gonadal lipid percentage: Lipid percentage in testis varied from its minimum value of 14.84 % to that of the maximum value of 20.91 % in the month of July to January respectively. The lipid content gradually increased from the July to that of January and reached to its maximum and then decreased to July. Lipid percentage in ovary was minimum 26.66 % in January to the maximum 43.03 % in July. Lipid content gradually increased from January to July and reached to its maximum value and then further decreased to January. Lipid percentage is minimum in the testis and maximum in the ovary in the month of July. In the month of January the lipid percentage is found to be maximum in the testis whereas minimum in the ovary. We also found that the monthly variation of lipid concentration in ovary was 1.79 times higher than that in the testis. (Table 2 and Fig.1)
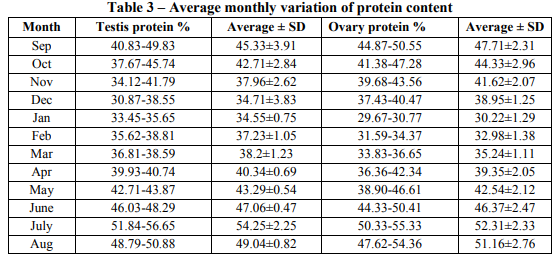
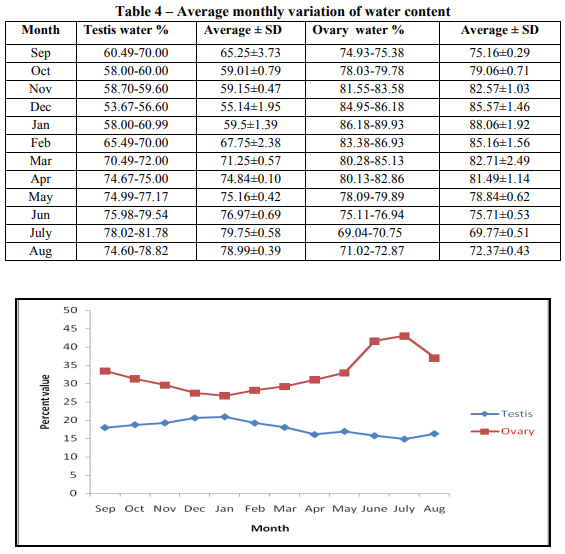
Gonadal protein percentage: Protein percentage in testis varied from its minimum 34.55 % to maximum 54.25 % in the month of January to July respectively. Protein percentage in ovary was minimum 30.22 % in January, maximum 52.31% in the July. In both gonads protein content gradually increased from January to July and reached to its maximum value and then further decreased to January. Monthly variation of protein content in the testis and the ovary was almost equal in percentage. (Table 3 and Fig. 2) Gonadal water percentage: Water percentage in testis has minimum value of 55.14 % to that of the maximum value of 79.75 % in the month of December to July respectively. The water content gradually increased from December to July and reached to its maximum value. Water percentage in ovary was minimum 69.77 % in July and maximum 88.06 % in January. Water content gradually increased from July to January and reached to its maximum value and then further decreased to July. Monthly variation of water concentration in ovary was 1.10 times higher than testis. (Table 4 and Fig. 3) Gonadal biochemical percentage and maturity: Since the gonadal maturity is explained by Gonado-somatic index (GSI), thus the percentage content of lipid, protein and water are plotted against Gonado-somatic index to find the effect of lipid, protein and water content on maturity. It is obvious from the plot that the water content (R2 = 0.664) is more responsible for maturation of testis in compare to lipid (R2 = 0.646) or protein (R2 = 0.264) content. For the maturation of ovary the lipid content (R2 = 0.601) is found to be more responsible as compare to protein (R2 = 0.172) or water content (R2 = 0.309). (Fig. 4 and Fig. 5)
DISCUSSION
Lipid helps in reproduction, maximize juvenile survivorship and growth Winemiller (1993). In the maturity phase of the male fish, spermatogenesis process was active; we observed that during the maturity period, the lipid concentration in testis has its minimum value. It is supported by a study in Clarias batrachus, in which the lipid concentration in testis had its minimum value in the spawning phase by Singh and Singh (1983). Henderson and Tocher (1987) Singh and Singh (1984) and Love (1970), obtained the similar observations that lipid concentration decreases as the maturity phase progress in testis. We observed that, lipid percentage in ovary has its minimum value in January (resting month) from where it increases continually to maximum in July (spawning month). It was also reported by Singh and Singh (1979), Shreni and Kalpana (1980) that there is an increase in the ovarian lipid during maturity phase in Hereropneustes fossilis. MacFarlane et al., (1993) Okuda (2001) and MacFarlane et. al. (1993), investigated the accumulated lipids in the developing ovaries. Shreni (1980) stated that the protein cycle in fishes synchronized with maturity of fishes. During maturity phase of fish, gonads increase many fold and attain large size and thus, in this period the protein concentration increases in the testis and ovary. In present study, during pre-maturity or resting phases the protein percentage was low while maximum during maturity phase in gonads (testis and ovary). These observations correlate positively to the results of Love (1970), John and Hameed (1995) who observed that resting gonads has minimum protein percentage. Bano (1977) studied that during the maturing phase protein is found to be increased and reached maximum which attributed to low metabolic activity. Macay and Tunison (1936), Jafri (1968) also noted the increased protein content in muscle which also attributed in increment with gonad maturity. Declining of protein in post-spawning phase was also reported by Somavanshi, (1983) in Garra mullya, and Luzzana et. al, (1996) in Coregonid bondella. In present study we observed that water percentage in testis was lowest in the resting month and highest during spawning month. Kingston and Venkataramani (1994) observed that he water content of testis in Selaroides leptoepis is higher during the peak maturity phase. Kumar et al., (2001) also found that there is an increase in water percentage of testis during active phase of reproductive cycle in Labeo rohita. Water percentage in ovary was highest in the resting month and lowest during spawning month. Similar study was done by Singh et. al. (2004) that the water content in ovary of female of Labeo rohita is decrease during the active reproductive phase. Singh and Nauriyal (1990) also found out the decrease ovarian water content and increase testicular water contain associated with maturity of cold water fishes Schizothorax richardsonii and Glypthorax pectinopterus.
CONCLUSIONS
In summary, we were able to find the July (Monsoon) was the spawning month of fish as well as monthly and seasonal variation of lipid, protein and water content in testis and ovary. We also obtained the months having minimum as well as maximum percentage value of these variables in testis and ovary. It was concluded that the lipid and water percentage was higher in ovary in compare to that of testis, while the protein content is almost same in both gonads. We also found that water content in testis and lipid content in ovary are more responsible for maturation than rest of the factors.
ACKNOWLEDGMENTS
The author acknowledges the LSRB-DRDO (R&D) New Delhi organization for financial assist and FRDL for Laboratory as well technical support.
References:
1. Bano, Y. (1977). Seasonal variation in the biochemical composition of Claris batrachus L. Proc. Ind. Acad. Sci., 85: 147-155.
2. CAMP (1998). Conservation assessment and management plan for freshwater fishes of India. Workshop report Zoo Outreach organization, Coimbatore/ CBSG and NBFGR, Lucknow, India, 1-158
3. Evans D.H. (1998). The physiology of fishes 2nd ed. CRC Press.Boca roton, New York. 441-464.
4. Folch, J., Lees, M. and Stanley, G.H.S (1957). A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem., 226: 497-509.
5. G.P. Dubey (1994). Endangered, vulnerable and rare fishes of west coast river systems of India. In: Threatened Fishes of India, P.V. Dehadrai, P. Das and S.R. Verma (Eds.). Natcon publication, India. 4: 77-95.
6. Henderson, R. J. & Tocher, D. R. (1987). The lipid-composition and biochemistry of freshwater fish. Progress in Lipid Research 26: 281-347.
7. Jafri A. K. & Khawaja D. K. (1968). Seasonal changes in biochemical composition of fresh water murrel Ophiocephallus punctatus (Bloch). Hydrobiologia, 32 (1-2): 206-218.
8. Jonh T. Sophy and Hameed M. Shanul (1995). Boichemical composition of Nemipterus japonicus and Nemipterus leptilepis in relation to maturity cycle. Fishery Tech 32(2): 102- 107
9. Kingston, S. and Venkataramani V.K. (1994). Biochemical composition yellow stripe scad, Selaroides leptoepis as a function of maturity stage and length fishery Tech., 31: 159-162.
10. Kumar, A., Singh I.J. and Ram R.N. (2001). Correlative cyclicity of certain biological indices and testicular development in Labeo rohita (Ham.) under tarai condition of Uttranchal. The Indian journal of Animal sciences (ICAR, New Delhi Publication), 71(11):1085-1087.
11. Love, R.M. (1970). The Chemical Biology of Fishes. Academic Press, Inc., London. 547.
12. Lowry, O.H., Rosebrough, N., Farr, A.L. and Randall, R.J. (1951). Protein measurement with Folin Phenol reagent. J. Biol. Chem., 193: 265-285.
13. Luzzana U. Serrini, G. Moretti V.M. Grimaldi P. Paleari M.A. and Valfre F. (1996). Seasonal variations in fat content and fatty acid composition of male and female Coreganid bondella from Lake Maggiore and Land locked shad from Lake Cemo (North Italy). J. Fish Boil. 48(3): 352-366.
14. Macay M. and Tunison A.V. (1936). Cortland Hatchery Report No. 5. N.Y. State Cons. Deptt. Us Bur of fish and Cornell Univ.
15. MacFarlane, R.B., Norton, E.C. and Bowers, M.J. (1993). Lipid dynamics in relation to the annual reproductive cycle in yellow tail rockfish, Sebastes flavidus. Canadian Journal of Fisheries and Aquatic Sciences, 50: 391- 401.
16. Okuda, N. (2001). The costs of reproduction to males and females of a paternal mouth brooding cardinalfish, Apogon notatus. Journal of Fish Biology, 58: 776-787.
17. P.S. Prasad (1994). Status paper on endangered, vulnerable and rare species of Bihar. In: Threatened Fishes of India. (P.V. Dehadrai, P. Das and S.R. Verma Eds.). Natcon publication, India. 4: 25-29.
18. Robertson, D. R. (1990). Differences in the seasonalities of spawning and recruitment of some small neotropical reef fishes. J. exp. mar. Biol. Ecol., 144: 49-62.
19. Shreni, D. Kalpana (1980). Seasonal variations in the chemical composition of cat fish. Heteropneustes fossilis (Bloch). Proc. Ind Acad Sci. (anim. Sci.), 89: 191-196.
20. Singh, A.K. and Singh, T.P. (1979). Seasonal fluctuation in total lipid and cholesterol content of ovary liver and blood serum in relation to annual sexual cycle in Hereropneustes fossilis (Bloch). Endocrinologie, 73: 47-54.
21. Singh, A.K., Kumar A., Singh I.J. and Ram R.N. (2004). Lipid and water content profiles in ovary and liver in a freshwater teleost, Labeo rohita during annual reproductive cycle in the tarai condition of Uttaranchal. Journal of Aquaculture in Tropics. 19(2): 137-144.
22. Singh, H.R. and Nauriyal, B.P. (1990). A comparative study of some biochemical constituents in the reproductive cycle of hill stream teleosts. Schizothorax richardsonii (Gray) & Glypthorax pectinopterus (Meleod). Proc. Natl. Acad. Sci., India, 16: 117-123.
23. Singh, I.J. and Singh, T.P. (1983). Annual changes in total gonadotropic potency in relation to gonadal activity in fresh water, Clarias batrachus. J. Inter Disci. Pl. Cycle. Res., 4: 227-239.
24. Singh, I.J. and Singh, T.P. (1984). Chang in gonadotropin, lipid cholesterol levels during annual reproductive cycle in the fresh water teleost, Cirrhinus mrigala (Ham.) Annales’D Endocrinologie, Paris, 45: 131-136.
25. Somvanshi (1983). Seasonal changes in biochemical composition of Hill stream fish Garra mullya, Ind. J. Fish. 30 (1).
26. Winemiller, K. O. (1993). Seasonality of reproduction by livebearing fishes in tropical rainforest streams. Oecologia, 95: 266-276.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License