IJCRR - 5(17), September, 2013
Pages: 47-53
Date of Publication: 12-Sep-2013
Print Article
Download XML Download PDF
HISTOPATHOLOGICAL PATTERN OF MALIGNANT BREAST TUMOURS AND CORRELATION OF CLINICOMORPHOLOGICAL FEATURES WITH MOLECULAR PROFILE (HORMONE RECEPTOR STATUS) IN KASHMIR
Author: Naila Nazir, Ruby Reshi, Sheikh Bilal, Summyia Farooq
Category: Healthcare
Abstract:Background: There is not much study done in our population group on the various types of breast tumours and the molecular profile (ER/PR status). Since Breast carcinoma incidence is increasing in our population the study was done to evaluate the different histopathological types, the hormone receptor status and there correlation with various clinicomorphological features in our population. Material and Methods: A two year prospective study was carried out on 50 patients with histologically confirmed invasive breast carcinomas which were further subjected to immunohistochemical assay (ER/ PR). Correlation with established risk factors age, tumour size, grade and histopathology were analysed. Results: ER and PR receptors determined by immunohistochemical method revealed ER+/PR+ in 52 %, ER+/PR- in 4 %, ER-/PR+ in 8% and ER-/PR- in 36% of the cases. Postmenopausal women showed a higher incidence of receptor positivity (77.27%) with increasing age. T2 tumours were more common (72%) as compared to T1 and T3 tumours. Receptor status was noted to be comparatively increased in larger sized tumours (88.9%). Infiltrating ductal carcinoma (NOS) was the commonest type (80%) with receptor positivity of 57.5%. Maximum tumours in the study were grade II (50%) which also showed maximum receptor positivity (64%) and the reactivity for the receptors was observed to decrease with increasing grade. Conclusion: ER/PR expression in breast cancers in the current study was found to be higher than the studies done in India/ Asia but still lower than studies done in west, even on Indian/ Asian immigrants to US and other western countries. This markedly lower receptor expression in Indian/ Asian studies is more likely due to preanalytic variables, threshold for positivity and interpretation criteria rather than genetic differences. So it is suggested that these variables need to be further identified and measures taken to rectify them so that a definite assessment of the receptor status can be done.
Keywords: Malignant, Breast tumours, Molecular profile, ER, PR.
Full Text:
INTRODUCTION
Breast cancer is a major medical problem in women and accounts for 22% of all female cancers with significant public health and social ramifications and is a leading cause of cancer death in women15,19. The number of cases worldwide has significantly increased since the 1970s, a phenomenon partly attributed to the modern lifestyles3,15. Breast cancer is the second most common cancer among women in India, following cancer of the uterine cervix. Presently, 75000 new cases are reported annually and account for 19-34% of all cancer cases among women nationally13,22. Breast cancer is a biologically heterogeneous disease and patients with the same diagnostic and clinical prognostic profiles can have markedly different clinical outcomes. Molecular profiling has provided biological evidence for heterogeneity of breast cancer through the identification of intrinsic subtypes. A crucial development in the evaluation of breast carcinoma has been the realization that the presence of estrogen and progesterone receptors (ER and PR) in the tumour tissue correlates well with response to hormone therapy and chemotherapy 2,11.
Since breast carcinoma is a common disease in this part of the country and a large number of breast carcinomas are diagnosed in our department, the present study was done to describe the morphology of malignant breast tumours- gross and histopathological types, study the molecular profile of breast tumours (estrogen receptor/ progesterone receptor expression) and the correlation between molecular profile of breast tumours and various clinicomorphological features.
MATERIAL AND METHODS
A two year prospective study was carried out from March 2011 to April 2013 on 50 patients in the post-graduate department of pathology, Government medical college Srinagar. Histopathologically confirmed invasive carcinoma cases were included in the study. Benign, in-situ lesions, sarcomas, and secondary lesions were excluded. Patient’s complete clinical data was recorded and the specimens received were fixed by keeping them in 10% formalin overnight. After fixing, gross examination of the specimen was done and findings recorded. Tissue sections about 1 cm apart were taken, put in stainless steel cassettes, labelled and kept in fixative for two to four hours. The tissue blocks were thoroughly washed with distilled water and the tissue was then dehydrated by passing through ascending grades of ethanol and then embedded in molten wax which was maintained in an oven at melting point of wax. A thin film of Mayer’s albumin was spread on clean glass slides and sections were placed on these slides and spread using hot water bath. After this, dewaxing was done by placing the slides in hot oven followed by passing through different grades of ethanol. The sections were stained routinely with Haematoxylin and Eosin and examined under the microscope. Grading of tumours was done according to modified Bloom-Richardson Grading System. IHC was performed by using the avidin-biotin complex peroxidase technique with the chromogen diaminobenzidine and antigen retrieval by heating specimen in microwave. For IHC Formalin fixed and paraffin embedded sections were cut and placed on a glass slides coated with 0.5% poly L-lysine. Endogenous peroxidase activity was blocked by placing slides in a mixture of methanol and hydrogen peroxide (9:1) for 20 minutes. Rabbit monoclonal antihuman estrogen receptor antibody - ER Clone SP1 and antihuman progesterone receptor antibody - PR Clone SP 2, Biocare were used. Sections were counter stained with Mayer’s Haematoxylin. ER and PR reactivity of invasive tumours was assessed. Sections from positive breast invasive ductal carcinomas were used as positive controls; negative controls were obtained by omitting the primary antibody. Scoring of ER and PR reactivity was done using Allred scoring system. All the data was subjected to statistical analysis.
RESULTS
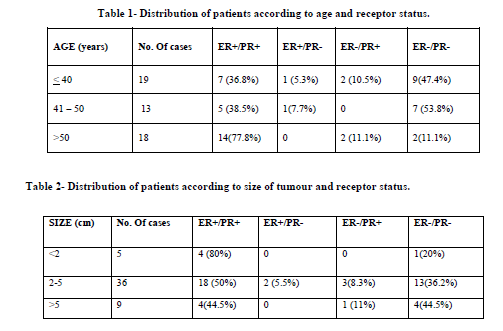
The age of the patients ranged from 27 – 85 years. Average age of patients was 49 years. Maximum number of patients belonged to age group of 31 - 50 years (56%). There were 3 male patients in our study. Out of 50 cases 48 cases were Modified Radical Mastectomies and 2 lumpectomy specimens, with left breast involved in 30(60%) of the cases and 20(40%) involved the right breast. Out of 47 female patients majority i.e. 42 (89.3%) were multiparous, where as 3 were nulliparous, 2 patients were unmarried. Out of 47 female patients 25 (53.2%) cases were pre-menopausal; where as 22(46.8%) were post-menopausal. Mean size of lesion was 3.68 cm, ranging from 1.5 cm to maximum of 10 cm. Most of the tumours were of the size between 2-5 cm (72%). IDC (Infiltrating ductal carcinoma) NOS (-not otherwise specified) was the predominant morphological type constituting 40(80%) of total cases. There was 1 case of Infiltrating Lobular Carcinoma, 2 cases of IDC with Paget’s disease of nipple, 1 case of Carcinoid, 1 case of Squamous cell carcinoma, 2 cases of Medullary Carcinoma, 1 case of Mucinous Carcinoma, 1 case of Carcinosarcoma and 1 case of Adenoid Cystic Carcinoma. According to Modified Bloom-Richardson Grading 9 (18%) cases were grade I, 25 (50%) cases were grade II and 16 (32%) cases were grade III. In our study of 50 cases 28 (56%) cases were ER positive, 30 (60%) cases were PR positive, 26 (52%) cases were both ER and PR positive, 18 (36%) cases were both ER and PR negative, 2 (4%) cases were ER positive and PR negative and 4 (8%) cases were ER negative and PR positive. In our series 77.8% cases above 50 years were found to be ER+/PR+ as compared to only 36.8% cases below 40 years. Among 13 cases in the age group of 41-50 years 38.5% cases were found to be ER+/PR+ (Table 1). In our study 72.7% cases were found to be ER+/PR+ among the postmenopausal cases as compared to only 36% in the premenopausal patients. 80% cases with tumour size less than 2 cm were found to be ER+/PR+ (Table 2). Among Histological type majority of the tumours showing ER/PR positivity were infiltrating ductal carcinoma-not otherwise specified type. In our series 64% of Grade II tumours were ER+/PR+ and decreased to 31.25% in Grade III tumours.
DISCUSSION
The mean age at presentation was 49 years; younger age at presentation as compared to western population was seen in our series which was in concordance with studies done in India2,20,25. Information on Receptor status was done in all 50 cases of which 28 (56%) cases were ER positive, 30 (60%) cases were PR positive. 26 (52%) cases were both ER and PR positive. So our patients show much better receptor positivity as compared with studies done in rest of Asia (Desai et al5 2000, Fatima et al8 2005, Kuraparthy et al14 2007, Shet et al25 2009) where positivity for ER ranges from as little as 31.6% and PR ranges from as little as 25.3% to maximum of <60%. This difference may be due to genetic differences, however other factors like threshold for positivity, are responsible for at least some of the difference.
However studies in west Dunnwald et al 200716, Kakarala et al17 2010 show ER positivity of more than 75% and PR positivity of more than 65% in Caucasians and ER, PR positivity of 70% and 60% respectively in Indian/Pakistani immigrant population to US. The shear sample size of these studies (155175 and 360933 respectively) lends credisence to their results which cannot be ignored. Results cannot be expected to vary so much between Asians in Asia and Asians in US as genetically they will be similar. What are reasons for this disparity?
Preanalytic variables, thresholds for positivity, and interpretation criteria seem to be the reasons of which first two are more important. Preanalytic variables which can lead to incorrect results include use of fixatives other than 10% neutral buffered formalin NBF (unless that fixative has been validated by the laboratory before offering the assay), biopsies fixed for intervals shorter than 6 hours or longer than 72 hours, samples where fixation is delayed for more than 1 hour, samples with prior decalcification using strong acids, and samples with inappropriate staining of internal assay controls (including intrinsic normal epithelial elements) or extrinsic assay controls7.
ER seems to be more vulnerable to preanalytic variables as earlier studies showed higher number of ER-/PR+ cases most of which subsequently turned out to be ER+/PR+ when repeated with a different set of antibodies using automated IHC18. Higher threshold for positivity as compared to recommended by American Society of Clinical Oncology/College of American Pathologists is another major reason for the disparity.
We compared receptor positivity with age at diagnosis and found that younger patients were less likely to be ER+/PR+ as compared to older patients, which is in concordance with studies done by Fisher6, Lisa k16, Graham A9, Colditz4, Ashba1, Desai5 and others.
CONCLUSION
ER and PR expression in breast cancers in the current study was found to be higher than studies done in India/Asia but still lower than studies done in west even on Indian/Asian immigrants to US and other western countries. Although receptor expression is lower in Indians/Asians compared to Caucasians but markedly lower receptor expression in Indian/Asian studies is more likely due to preanalytic variables, thresholds for positivity and interpretation criteria rather than genetic differences. So it is suggested that these variables need to be further identified and measures taken to rectify them so that a definite assessment of receptor status in our population can be done.
ACKNOWLEDGEMENTS
We thank Dr. Adil, Dr. Rohi, Dr. Salma, Dr. Sheema and Dr. Mahnaaz for helping us in compiling the data. We also thank Dr. Muzamil Ahmad Baba for his technical assistance.

References:
- Ashba J, Traish AM. Estrogen and progesterone receptor concentrations and prevalence of tumour hormonal phenotypes in older breast cancer patients. Cancer Detect Prev. 1999;23(3):238-44.
- Azizun-nisa et al. Comparison of ER,PR and HER-2/neu (C-erb B 2) reactivity pattern with histologic grade, tumor size and lymph node status in breast cancer. Asian Pac J Cancer Prev.2008 Oct-Dec;9(4):553-6.
- Breast Cancer: Statistics On Incidence, Survival, And Screening". Imaginis Corporation. 2006. Retrieved 2006-10-09.
- Colditz GA: Relationship between estrogen levels, use of hormone replacement therapy, and breast cancer. J. Natl Cancer Inst. 90, 814–823 (1998).
- Desai Sb, Moonim Mt, Gill Ak, Punia Rs, Naresh Kn, Chinoy Rf. Hormone Receptor Status Of Breast Cancer In India: A Study Of 798 Tumors. Breast 2000; 9: 267-70
- Edwin R. Fisher, Md, Carol K. Redmond, Scd, Hannen Liu, Md, Mph, Howard Rockette, Phd,Bernard Fisher, Md And Collaborating Nsabp Investigators Correlation Of Estrogen Receptor And Pathologic Characteristics Of Invasive Breast Cancer. Cancer 45:349-353, 1980
- Elizabeth et al. American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations for Immunohistochemical Testing of Estrogen andProgesterone Receptors in Breast CancerArch Pathol Lab Med. 2010;134:907–922
- Fatima S, Faridi N, Gill S.Breast Cancer: Steroid Receptors And Other Prognostic Indicators.J Coll Physicians Surg Pak. 2005 Apr;15(4):230-3.
- Graham A. Colditz, Bernard A. Rosner, Wendy Y. Chen, Michelle D. Holmes,Susan E. Hankinson Risk Factors for Breast Cancer According to Estrogen and Progesterone Receptor Status Journal of the National Cancer Institute, Vol. 96, No. 3, February 4, 2004 218-28
- Grazia Arpino, Heidi Weiss, Adrian V. Lee. Estrogen Receptor – Positive, Progesterone Receptor–Negative Breast Cancer: Association With Growth Factor Receptor Expression and Tamoxifen Resistance Journal of the National Cancer Institute, Vol. 97, No. 17, September 7, 2005.
- Hawkins RA, Roberts MM, Forrest APM. Estrogen receptors and breast cancer. Current status. Br J Surg 1980, 67:162 -165).
- Horita H, Yamaguchi A, Hirose K et al. Prognostic factors affecting disease free survival rate following surgical resection of primary breast cancer. Eur J Histochem 2001; 45:73-84.
- Indian Council Of Medical Research. Biennial Report (1989). New Delhi, (India.). (Badwe Ra, Mitra I, Desai Pb (1990).
- Kuraparthy S. Et al. Epidemiology and patterns of care for invasive breast carcinoma at a community hospital in Southern India World Journal of Surgical Oncology 2007, 5:56 doi:10.1186/1477-7819-5-56
- Laurance, Jeremy (2006-09-29). "Breast Cancer Cases Rise 80% Since Seventies". The Independent. Retrieved 2006-10-09.
- Lisa K Dunnwald, Mary Anne Rossing And Christopher I Li Hormone Receptor Status, Tumor Characteristics, And Prognosis: A Prospective Cohort Of Breast Cancer Patients. Breast-Cancer-Research.2007Com /Content /9/1/R6)
- Madhuri Kakarala, Laura Rozek, Michele Cote, Samadhi Liyanage And Dean E Brenner Breast Cancer Histology And Receptor Status Characterization In Asian Indian And Pakistani Women In The U.S. – A Seer Analysis. Kakarala Et Al. Bmc Cancer 2010, 10:191
- Navani S And Bhaduri AsHigh Incidence Of Estrogen Receptor Negative Progesterone Receptor Positive Phenotype In Indian Breast Cancer: Fact Or Fiction? Indian J Pathol Microbiol.2005 Apr;48(2):199_201
- Parkin Dm, Bray F, Ferlay J, Pisani P (2001). Estimating The World Cancer Burden: Globocan 2000. Int J Cancer, 94, 153-6.-
- Rao R, Kuerer H, Cristofanilli M, Brigilio K, Shukla S, Rao M, Krishnamurthy S:Breast cancer in the asian Indian population of the United States: a call for screening and education. Breast J 2008, 14(4):402-403.
- Saxena S, Rekhi B, Bansal A, Et Al (2007). Clinico-Morphological Patterns Of Breast Cancer Including Family History In A Newdelhi Hospital, India-A Cross-Sectional Study. World J Surg Oncol, 3, 67.)
- Siddiqi M, Sen U, Mondal T, Et Al (2001). Cancer Statistics From Non-Icmr Registries: Population Based Registries, Crab (Cancer Registry Abstract). Newsletter of the National Cancer Registry Project Of India. Pp. 47–59.)
- Sorlie T, Tibshirani R, Parker J, Et Al (2003). Repeated Observation Of Breast Tumor Subtypes In Independent Gene Expression Data Sets. Proc Natl Acad Sci USA, 100, 8418-23.
- Sotiriou C, Neo Sy, Mcshane Lm, Et Al (2003). Breast Cancer Classification And Prognosis Based On Gene Expression Profiles From A Population-Based Study. Proc Natl Acad Sci USA, 100, 10393-8.
- Tanuja Shet, Atin Agarwal, Mandar Nadkarni, Mahendra Palkar, Rohini Havaldar, Vani Parmar, Rajendra Badwe, R. F. ChinoyHormone Receptors Over The Last 8 Years In A Cancer Referral Centre In India: What Was And What Is? Indian J Of Pathol And Microbiology -52(2), April-June 2009:171-174
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License