IJCRR - 5(17), September, 2013
Pages: 16-23
Date of Publication: 12-Sep-2013
Print Article
Download XML Download PDF
AUTOIMMUNE RETINOPATHY-A REVIEW
Author: Devendra Pratap Singh Rajput, Priti Singh, S. B. Gupta, Samarth Shukla, Sourya Acharya, Rashmi Kumar
Category: Healthcare
Abstract:Autoimmune retinopathy is a rare autoimmune disease that primarily affects retinal photoreceptor function. It mainly presents in the fifth and sixth decades. Three main forms of autoimmune retinopathy (AIR) have been identified: cancer-associated retinopathy (CAR), melanoma-associated retinopathy (MAR), and non-neoplastic autoimmune retinopathy (npAIR). In this chapter, the term AIR will be used to encompass all three disorders. Patients typically present with a sudden onset of photopsia, rapid visual loss, and abnormal electroretinograms (ERGs). Different types of AIR present with similar clinical features and it requires extensive work up to rule out other differential diagnosis. Pt presents with poor visual prognosis that may be due to delayed diagnosis and delay in initiation of treatment. Different treatment modalities have been tried, including systemic immunosuppression with steroid and steroid-sparing agents, intravenous immunoglobulin, and plasmapheresis, with variable results. Different types of antiretinal antibodies have been found in these patients with autoimmune retinopathy such as antibodies to recoverin, \a-enolase and transducin-\a, but seronegative disease is also common. A lot of research work has been done in this field to understand the pathophysiological mechanisms that is responsible for autoimmune retinopathy, but than also understanding about this rare disorder is limited. In this review we have tried to summarize the pathogenic mechanism, clinical features, investigation, differential diagnosis, treatment and prognosis of autoimmune retinopathy.
Keywords: Autoimmunity, Retinopathy, Autoantibody
Full Text:
INTRODUCTION
Auto immunity is a condition in which ones own tissues are prone to be affected by deleterious effects of the immunological system. Autoimmune retinopathy occurs when antigens trigger an immune response, which produces antibodies those cross reacts with a retinal protein. Autoimmune retinopathy represent an important cause of an otherwise unexplained acute or sub-acute vision loss in adults. These forms of retinal disease result from a presumed immunological process affecting the retina by auto antibodies directed against retinal antigens(1-3).
Autoimmune retinopathies can occur:
1. Rarely as primary autoimmune retinopathy.
2. More commonly as.
a. Cancer associated retinopathy (CAR)
b.Retinopathies secondary to various autoimmune reactions.
Cancer associated retinopathy is the term that has been used for the retinal degeneration first described by Sawyer and associates [4] in 1976 as a distant effect of cancer. Paraneoplastic retinopathy, a term first used by Klingele and associates in 1984(5) has become the more general term used for any of a number of autoimmune retinopathy associated with a malignant tumor. Autoimmune retinopathy is the preferred term for an acquired, presumed immunologically mediated retinal degeneration with symptoms resembling paraneoplastic retinopathy(2).
The etiology and source of antigenic stimulation vary but are largely unknown. It is possible that the disease is triggered by molecular mimicry between retinal proteins and presumed viral or bacterial proteins or by the acquired alteration of host tissues or antigen so that the autoimmunity is induced against retinal proteins. Multiple retinal proteins have been found to be antigen including recoverin , enolase , arestin , transdusin TUPL I , neurofilament protein , heat shock protein ,70 PNR and as yet unidentified bipolar cell antigen causing melanoma associated retinopathy (MAR syndrome)(6) .
Autoimmune retinopathies are ophthalmic disorders in which autoantibody damage retina and its components causing progressive vision loss. Autoimmune retinopathy typically presents in the fifth and sixth decades with rapidly progressive, bilateral, painless visual deterioration(7).
Specific forms of autoimmune retinopathies that have been identified include cancer associated retinopathy (CAR)(4,8), melanoma associated retinopathy (MAR)[6] , anti-enolase retinopathy[2], anti-carbonic anhydrase retinopathy and cancer associated cone dysfunction(9).
Some patients of secondary autoimmune retinopathy had associated systemic autoimmune diseases such as rheumatoid arthritis, grave’s disease, systemic lupus erythematosus and antiphospholipid antibody syndrome(10).
EPIDEMIOLOGY
Autoimmune retinopathy is an uncommon disorder, exact prevalence not known. It usually affect older adults, but patients as young as three years have been described with no sex predilection(11,12). Cancer associated retinopathy is most common form of autoimmune retinopathy. The malignancy most commonly associated with disorders is small-cell lung cancer, followed by gynecological breast cancers. Some cases have been reported with Hodgkin’s lymphoma, pancreatic and colon cancers (11,13). MAR appears to be increasing in frequency relative to CAR, perhaps because of a decrease in cases of lung cancer(11).
CLINICAL FEATURES
Autoimmune retinopathy typically presents in the fifth and sixth decades with rapidly progressive, bilateral, painless visual deterioration but an unremarkable fundus examination(7). Patients typically present with sudden onset of photopsia, rapid visual loss, and abnormal electroretinograms (ERG)(14). Bilateral vision loss as a result of both rod and cone dysfunction in CAR may occur over a period of months, visual symptoms may precede diagnosis of the systemic malignancy(15).
The triad of photosensitivity, ring scotoma, and a reduced caliber of the retinal arteriole along with undetectable signals in ERG are specific manifestations of CAR(16). MAR is characterized by shimmering, flickering or pulsating photopsias and usually occurs in the patients with cutaneous melanoma(16).
Besides glare sensitivity and flashing lights, a rapidly progressive, often asymmetric visual loss may occur. Although paracentral and mid-peripheral scotomas can be found frequently, visual field defects are often quite heterogeneous (17).
Individuals with cone involvement have
- Photosensitivity (light sensitivity)
- Prolonged glare after light exposure (hamarolopia)
- Reduced visual acuity and loss of vision.
Patients with rod involvement have
- Difficulty in seeing in dim lighting (Nyctalopia).
- Prolonged dark adaptation.
- Peripheral field vision loss.
Signs
- Decreased central visual acuity
- Visual field defects (central, paracentral or equatorial scotomas)
- Alternate pupillary defect if asymmetric involvement.
- Defective color vision.
Fundus Findings
Fundus can appear normal initially but with progression there is evidence of retinal degenerations (Retinal pigment epithelium RPE thickening and mottling, attenuation of the arterioles, optic nerve pallor. Cystoid macular edema (CME) has been reported in patients with non paraneoplastic retinopathy (npAIR) but is less common with CAR (18,19).
As reported by Keltner et al, fundus findings in 43 patients with MAR were as follows: 19 (44%) patients had normal fundus findings at presentation, 13 (30%) had vascular attenuation, and 12 (28%) had RPE changes. Vitreous cells were present in 13 (30%) patients, and 10 (23%) had optic disc pallor (11, 20)
Investigations and Diagnosis
All the patients who presented with unexplained loss of central vision, visual field defects, and/or photopsia are diagnosed with AIR based on clinical features, ERG findings, serum antiretinal antibody analysis and OCT testing for macula (10).
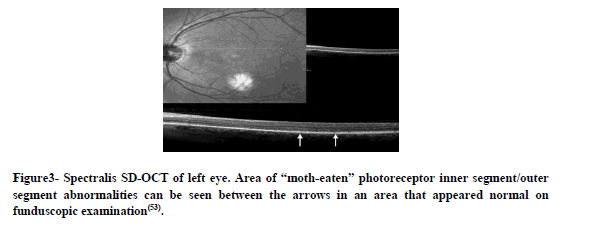
On OCT, patients show outer retinal abnormalities and/or decreased macular thickness. In Macular OCT reduced central macular, foveal thickness, loss of the photoreceptor layer or disruption of the photoreceptor outer and inner segment junction was noted(10)
Figure3
It should also be noted that antiretinal antibodies may be present in the normal population and their presence does not necessarily indicates retinopathy(10).For example, while anti recoverin autoantibody is not typically present in the normal population, the frequency of anti-α-enolase autoantibody is approximately 10% in healthy subjects; however this is not well defined for other anti-retinal auto antibodies(21,22).
It was found that autoantibodies against retinal proteins from patients with retinopathy were cytotoxic to retinal cells, in contrast to those from healthy subjects, probably through recognition of additional unique regions on their target retinal antigen(23).
Antibody Testing and their cytotoxic effects can be assessed with western blot, ELISA, immunocytochemistry, cytotoxicity assay for acute recovering antienolase antibodies assay[24].
The literature varies in diagnostic criteria for AIR and firm establishment of this diagnosis is challenging.
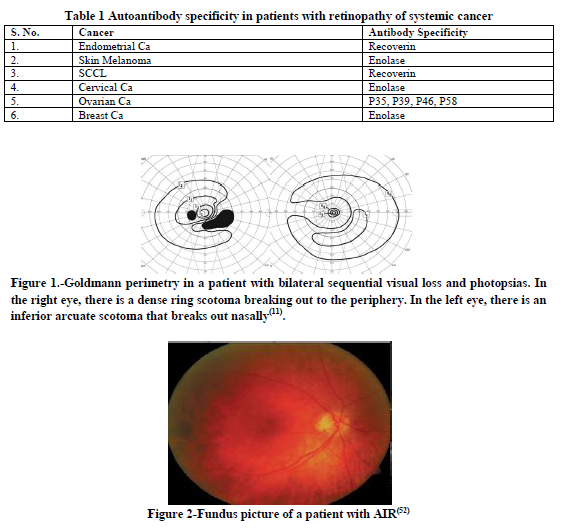
There have been different antibodies isolated against many specific retinal proteins in patient with autoimmune retinopathies. Patients with CAR possess autoantibodies, including recovering (23KDa), α-enolase(46KDa)(21,25). Other autoantibodies against retinal proteins have also been reported such as neurofilament proteins, heat-shock protein 70, TULPI protein, 40KDa insoluble protein(21,25-30).Auto antibodies binding to bipolar cells have been linked to the melanoma- associated retinopathy (MAR) syndrome(31-33)
Table 1
ELECTRORETINOGRAM
- Typically, the responses in the ERG are markedly reduced, but normal ERGS are also described (17).Full field ERG are almost always abnormal, attenuated or absent photopic and scotopic response. IN CAR where mainly the cones are affected, full filed ERG could be normal but multifocal ERG will be abnormal.
DIFFERENTIAL DIAGNOSIS OF AUTOIMMUNE RETINOPATHY
- Retrobulbar optic neuropathy.
- Toxic nutritional optic neuropathy or hereditary optic neuropathy.
- In malignancy unexplained visual loss may be due to infiltration of malignant cell around optic nerves metastasis to orbit and optic neuropathy due to chemotheraputic agents
- Acute Zonal occult outer retinopathy (AZOOR).
TREATMENT
Treatment of primary disease should be done in conjunction with a physician and an oncologist. Long term immune suppression is the main therapy. Immunosuppression has been used to treat AIR with mixed results. Sawyer et al treated 1 of the original 3 patients with CAR with prednisone but saw no improvement34. Keltner et al reported the first patient with CAR responsive to corticosteroid therapy35. Since then, there have been numerous case reports in the literature using short-course high-dose intravenous methylprednisolone or oral prednisone. Plasmapheresis, when used alone, led to no improvement36, when used with prednisone, vision improved in 1 patient37. Guy and Aptsiauri reported improvement in 2 of 3 patients treated with intravenous immunoglobulin and stabilization in the third38. Espandar et al recently reported stabilization of CAR with alemtuzumab therapy39.
Various treatment modalities have been tried in patients with CAR, including oral and intravenous steroids, plasmapheresis, IVIg, rituximab, azathioprine, cyclosporine, and mycophenolate mofetil[40-43]. Despite treatment with these systemic medications, it is not unusual to have a progressive decline in vision with this disease 44. Serial intravitreal injection of triamcinolone may be beneficial for maintenance of vision in patients with CAR 44.
PROGNOSIS
Treatment may provide mild to moderate transient visual acuity improvement. But overall the visual prognosis remains poor. In cases of CAR, systemic cancer treatment usually do not lead to visual improvement However prognosis depends on their underlying malignancy (16).
DISCUSSION
The diagnosis of autoimmune retinopathy remains extremly challenging. Patient has to undergo extensive neurological and neuro- ophthalmogical evaluation it also should be noted that antiretinal antibodies may be present in the normal population and their presence dose not necessarily indicate retinopathy.
Different mechanism of cell damage have been suggested for anti recoverin (45,46) and anti-enolase antibodies(47,48) predominantly resulting in apoptosis of retinal cells, therefore it appears that apoptosis may be a common pathway for retinal autoantibody induced retinal degeneration.
The evidence supporting the effects of antibodies on retinal cells are the following findings:
a) Autoantibodies against recoverin specifically labeled retinal photoreceptor cells and were internalized by cells causing their apoptotic death[ 49].
b) In CAR patients, autoantibodies against α-enolase induced the apoptotic death of retinal cells, and in glaucoma patients, autoantibodies against γ-enolase labeled retinal ganglion cells and induced their death through apoptosis [50,51]
Independent of specificity, autoantibody-induced apoptosis is a pathway to retinal death in AR.
However the pathogenic mechanisms of retinopathies are complex and our understanding of AR is still incomplete. Further studies are necessary to identify anti-retinal autoantibodies, to test their pathogenic potentials through in vivo and in vitro methods, and to define clinical and electrophysiological indicators for seropositive patients.
ACKNOWLEDGEMENT
Authors acknowledge the great help received from the scholars whose articles have been cited and included in references of this manuscript. The authors are also grateful to authors / editors / publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed. Authors are grateful to IJCRR editorial board members and IJCRR team of reviewers who have helped to bring quality to this manuscript.
References:
- Ohguro H, Yokoi Y, Ohguro I, et al. Clinical and immunologic aspects of cancer-associated retinopathy. Am J Ophthalmol. 2004; 137(6):1117–1119. [PubMed: 15183799]
- Weleber RG, Watzke RC, Shults WT, et al. Clinical and electrophysiologic characterization of paraneoplastic and autoimmune retinopathies associated with antienolase antibodies. Am J Ophthalmol. 2005; 139(5):780–794. [PubMed: 15860281]
- Whitcup SM, Vistica BP, Milam AH, Nussenblatt RB, Gery I. Recoverin-associated retinopathy: a clinically and immunologically distinctive disease. Am J Ophthalmol. 1998; 126(2):230–237. [PubMed: 9727517]
- Sawyer RA, Selhorst JB, Zimmerman LE, Hoyt WF. Blindnesscaused by photoreceptor degeneration as a remote effect of cancer. Am J Ophthalmol 1976;81:606–613
- Klingele TG, Burde RM, Rappazzo JA, Isserman MJ, BurgessD, Kantor O. Paraneoplastic retinopathy. J Clin Neuroophthalmol 1984;4:239 –245.
- Berson EL, Lessell S. Paraneoplastic night blindness withmalignant melanoma. Am J Ophthalmol 1988;106:307–311.
- Braithwaite T, Vugler A, Tufail A. Autoimmune retinopathy. . Ophthalmologica. 2012;228(3):131-42.
- Thirkill CE, Roth AM, Keltner JL. Cancer-associated retinopathy. Arch Ophthalmol. Mar 1987;105(3):372-5. [Medline].
- Adamus G, Karren L. Autoimmunity against carbonic anhydrase II affects retinal cell functions in autoimmune retinopathy. J Autoimmun. Mar 2009;32(2):133-9. [Medline].
- Azin Abazari, Souha S. Allam, Grazyna Adamus, And Nicola G. Ghazi, Optical Coherence Tomography Findings in Autoimmune Retinopathy, Am J Ophthalmol. 2012 April ; 153(4): 750–756.e1.
- Raj K Maturi, MD; Chief Editor: Hampton Roy Sr, MD, Cancer Associated and Related Autoimmune Retinopathies, emedicine.medscape.com/article/1227724.
- Ko AC, Hernández J, Brinton JP, Faidley EA, Mugge SA, Mets MB. Anti-?-enolase autoimmune retinopathy manifesting in early childhood. Arch Ophthalmol. Dec 2010;128(12):1590-5. [Medline].
- Adamus G. Autoantibody targets and their cancer relationship in the pathogenicity of paraneoplastic retinopathy. Autoimmun Rev. Mar 2009;8(5):410-4. [Medline].
- Heckenlively JR, Ferreyra HA. Autoimmune retinopathy: a review and summary, Semin Immunopathol. 2008 Apr;30(2):127-34.[article8]
- Khan N, Huang JJ, Foster CS. Cancer associated retinopathy (CAR): An autoimmune-mediated paraneoplastic Syndrome, Semin Ophthalmol. 2006 Jul-Sep;21(3):135-41.
- Ikawa M, Kuriyama M. Paraneoplastic retinopathy and optic neuropathy. Brain Nerve. 2010 Apr;62(4):371-6.
- Sobottka B, Schlote T, Besch D, Djelebova T, Wilhelm H, Zrenner E.et al. Carcinoma-associated retinopathy: a review with clinical examples. . Klin Monbl Augenheilkd. 2000 Jan;216(1):17-24.
- Ferreyra HA, Jayasundera T, Khan NW, He S, Lu Y, Heckenlively JR (2009) Management of autoimmune retinopathies with immunosuppression. Arch Ophthalmol 127:390–397.
- Nancy Huynh and Yevgeniy Shildkrot and Ann-Marie Lobo and Lucia Sobrin. Intravitreal triamcinolone for cancer-associated retinopathy refractory to systemic therapy. J Ophthal Inflamm Infect (2012) 2:169–171
- Keltner JL, Thirkill CE, Yip PT. Clinical and immunologic characteristics of melanoma-associated retinopathy syndrome: eleven new cases and a review of 51 previously published cases. J Neuroophthalmol. Sep 2001;21(3):173-87. [Medline].
- Adamus G, Aptsiauri N, Guy J, Heckenlively J, Flannery J, Hargrave PA. The occurrence of serum autoantibodies against enolase in cancer-associated retinopathy. Clin Immunol Immunopathol. 1996; 78(2):120–129. [PubMed: 8625554].
- Gitlits VM, Toh BH, Sentry JW. Disease association, origin, and clinical relevance of autoantibodies to the glycolytic enzyme enolase. J Investig Med. 2001; 49(2):138–145.
- Adamus G, Amundson D, Seigel GM, Machnicki M. Anti-enolase alpha autoantibodies in cancerassociated retinopathy: epitope mapping and cytotoxicity on retinal cells. J Autoimmun. 1998; 11(6):671–677. [PubMed: 9878089].
- Grazyna Adamus*1,2, Gaoying Ren1 and Richard G Weleber2. Autoantibodies against retinal proteins in araneoplastic and autoimmune retinopathy. BMC Ophthalmology 2004, 4:5.
- Thirkill CE, FitzGerald P, Sergott RC, Roth AM, Tyler NK, Kaltner JL: Cancer-associated retinophaty (CAR syndrome) with antibodies reacting with retinal, optic-nerve, and cancer cells. New England Journal of Medicine 1989, 321:1589-1594.
- Murphy MA, Thirkill CE, Hart WM: Paraneoplastic retinopathy: a novel autoantibody reaction with small cell carcinoma. J Neuroophthalmol 1997, 17:77-83. .
- Ohguro H, Ogawa K, Nakagawa T: Recoverin and Hsc 70 are found as autoantigens in patients with cancer-associated retinopathy. Invest Ophthalmol Vis Sci 1999, 40:82-89. .
- Ohguro H, Ogawa K, Maeda T, Maeda A, Maruyama I: Cancer-associated retinopathy induced by both anti-recoverin and antihsc70 antibodies in vivo. Invest Ophthalmol Vis Sci 1999, 40:3160-3167. .
- Yoon YH, Cho EH, Sohn J, Thirkill CE: An unusual type of cancerassociated retinopathy in a patient with ovarian cancer. Korean J Ophthalmol 1999, 13:43-48. .
- Peek R, Dijkstra BG, Meek B, Kuijpers RW: Autoantibodies to photoreceptor membrane proteins and outer plexiform layer in patients with cancer-associated retinopathy. Clin ExpImmunol 2002, 128:498-503. .
- Milam AH, Saari JC, Jacobson SG, Lubinski WP, Feun LG, Alexander KR: Autoantibodies against retinal bipolar cells in cutaneous melanoma-associated retinopathy. Invest Ophthalmol Vis Sci 1993, 34:91-100.
- Keltner JL, Thirkill CE: The 22-kDa antigen in optic nerve and retinal diseases. J Neuroophthalmol 1999, 19:71-83.
- Keltner JL, Thirkill CE, Yip PT: Clinical and immunologic characteristics of melanoma-associated retinopathy syndrome: eleven new cases and a review of 51 previously published cases. J Neuroophthalmol 2001, 21:173-187.
- Sawyer RASelhorst JBZimmerman LEHoyt WF Blindness caused by photoreceptor degeneration as a remote effect of cancer. Am J Ophthalmol 1976;81 (5) 606- 613]
- Keltner JLRoth AMChang RS Photoreceptor degeneration: possible autoimmune disorder. Arch Ophthalmol 1983;101 (4) 564- 569]
- Thirkill CEFitzGerald PSergott RCRoth AMTyler NKKeltner JL Cancer-associated retinopathy (CAR syndrome) with antibodies reacting with retinal, optic-nerve, and cancer cells. N Engl J Med 1989;321 (23) 1589- 1594]
- Murphy MAThirkill CEHart WM Jr Paraneoplastic retinopathy: a novel autoantibody reaction associated with small-cell lung carcinoma. J 2Neuroophthalmol 1997;17 (2) 77- 83]
- Guy JAptsiauri N Treatment of paraneoplastic visual loss with intravenous immunoglobulin: report of 3 cases. Arch Ophthalmol 1999;117 (4) 471- 477]
- Espandar L, O'Brien S, Thirkill C, Lubecki LA, Esmaeli B. Successful treatment of cancer-associated retinopathy with alemtuzumab. J Neurooncol. Jul 2007;83(3):295-302.
- Ferreyra HA, Jayasundera T, Khan NW, He S, Lu Y, Heckenlively JR (2009) Management of autoimmune retinopathies with immunosuppression. Arch Ophthalmol 127:390–397
- Guy J, Aptsiauri N (1999) Treatment of paraneoplastic visual loss with intravenous immunoglobulin. Arch Ophthalmol 127:612–614
- Shildkrot Y, Sobrin L, Gragoudas ES (2011) Cancer-associated retinopathy: update on pathogenesis and therapy. Sem Ophthalmol 26:321–328
- Mahdi N, Faia LJ, Goodwin J, Nussenblatt RB, Sen HN (2010) A case of autoimmune retinopathy associated with thyroid carcinoma. Ocul Immunol Inflamm 18:322–32
- Nancy Huynh and Yevgeniy Shildkrot and Ann-Marie Lobo and Lucia Sobrin-J Ophthal Inflamm Infect (2012) 2:169–171
- Adamus G, Machnicki M, Elerding H, Sugden B, Blocker YS, Fox DA. Antibodies to recoverin induce apoptosis of photoreceptor and bipolar cells in vivo. J Autoimmun. 1998; 11(5):523–533. [PubMed: 9802939].
- Maeda T, Maeda A, Maruyama I, et al. Mechanisms of photoreceptor cell death in cancerassociated retinopathy. Invest Ophthalmol Vis Sci. 2001; 42(3):705–712. [PubMed: 11222531].
- Magrys A, Anekonda T, Ren G, Adamus G. The role of anti-alpha-enolase autoantibodies in pathogenicity of auto-immune-mediated retinopathy. J Clin Immunol. 2007; 27(2):181–192. [PubMed: 17235687].
- Ren G, Adamus G. Cellular targets of anti-alpha-enolase autoantibodies of patients with autoimmune retinopathy. J Autoimmun. 2004; 23(2):161–167. [PubMed: 15324934].
- Adamus G, Machnicki M, Seigel GM: Apoptotic retinal cell death induced by autoantibodies of cancer associated retinopathy. Invest Ophthalmol Vis Sci 1997, 38:283-291].
- Adamus G, Amundson D, Seigal GM, Machnicki M: Anti-enolase alpha autoantibodies in cancer-associated retinopathy: epitope mapping and cytotoxicity on retinal cells. J Autoimmun 1998, 11:671-677
- Maruyama I, Ohguro H, Ikeda Y: Retinal ganglion cells recognizedby serum autoantibody against gamma- enolase found in glaucoma patients. Invest Ophthalmol Vis Sci 2000, 41:1657-1665.
- Dr Kathryn L. Pepple, PhD, Dr Prithvi Mruthyunjaya OphthalmologyTimesEurope Volume 9, Issue 5.
- Kimberly E. Stepien, MD,* Dennis P. Han, MD, Jonathan Schell, MD, Pooja Godara, MD, Jungtae Rha, PhD, and Joseph Carroll, PhD. Trans Am Ophthalmol Soc. 2009 December; 107: 28–33.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License