IJCRR - 5(20), October, 2013
Pages: 01-07
Date of Publication: 02-Nov-2013
Print Article
Download XML Download PDF
SIDEROPHORE PRODUCTION BY THE ISOLATES OF FLUORESCENT PSEUDOMONADS
Author: Sujatha N., Ammani K.
Category: General Sciences
Abstract:Production of secondary metabolites including siderophores is yet another mechanism by which fluorescent pseudomonads acts as a biocontrol agent. Siderophores are secreted under iron limitation and have a very high affinity for ferric iron. The resulting ferric-siderophore complex is unavailable to other organisms, but the producing strain can utilize this complex via a specific receptor in its outer cell membrane. In this way, siderophore producing fluorescent Pseudomonads may restrict the growth of phytopathogens. In the present study the siderophore production by the isolated strains of fluorescent pseudomonads has been reviewed. Of the 22 isolates which showed the abilty to produce siderophores, 6 isolates were shown to produce catecholate type of siderophores and the remaining 16 isolates showed the presence of hydroxamate siderophores.
Keywords: Catecholate siderophores, hydroxamate siderophores, FeCl3 Test, Arnow’s Test, Tetrazolium Test, CAS assay and Spectrophotometric assay.
Full Text:
INTRODUCTION
Iron, is a vital micronutrient needed for metabolism by many plants, due to its diverse role in the biosynthesis of chlorophyll, redox reactions and many physiological activities. The availability of iron to plants in neutral and alkaline soils are very limited. Due to iron starvation, the productivity of the crops is decreasing and this would lead to a severe damage to the economy. Microbial siderophores are one of the major sources of iron in plants. Siderophores are low molecular-weight compounds which are produced under iron-limiting conditions and change iron to soluble and absorbable form for plants and microoganisms. Siderophore producing Pseudmonads play vital role in stimulating the growth of the plant and in controlling phytopathogens.
MATERIALS AND METHODS
Siderophore Production
The cultures were inoculated in King’s B broth medium and incubated for 48 h at room temperature. After the incubation period, the cultures were centrifuged at 10,000 rpm for 15 min. The cell-free culture filtrate was used for the following tests for siderophore production.
CAS method
Production of siderophore was determined by Chromazurol Sulphonate (CAS) agar method [1]. The bacterial inoculum was spotted onto the center of a CAS agar plate. After incubation at 28°C for 5 days, siderophore production was assayed by the change in the colour of the medium from blue to orange.
FeCl3 Test [2]
To 0.5 ml of culture filtrate, 0.5 ml of 2% aqueous FeCl3 solution was added and examined for the appearance of orange or reddish brown colour which was positive indication of siderophore production.
Spectrophotometric assay
Cultured bacterial cells were harvested by centrifuging at 10,000 rpm for 10 minutes. The supernatant was subjected to Neiland’s spectrophotometric analysis to confirm siderophore production.
The hydroxamate nature of siderophore was detected by Neilands spectrophotometric assay [3] where a peak between 420-450 nm on addition of 3ml of freshly prepared 2% aqueous solution of FeCl3 to 1 ml of supernatant indicated presence of Ferrate hydroxamate. Catecholate nature of siderophore was detected by the method of Jalal and Vander Helm [3] using spectrophotometric assay where a peak at 490 nm on addition of 2% aqueous solution of FeCl3 to 1ml of supernatant indicated the presence of siderophores of catecholate nature.
Determinations of the chemical nature of the siderophores were also done by Arnow’s test and Tetrazolium test.
Arnow’s Test for catecholate siderophores [4]
To 1 ml of culture filtrate, 1 ml of 0.5 N HCl, and 1 ml of nitrite molybdate reagent were added. Then 1 ml of 1N NaOH was added. The formation of red coloured solution which was the indication of the presence of catechol type of siderophore was examined.
Preparation of nitrite molybdate reagent
To 10 g of sodium nitrite was added 10 g of sodium molybdate. The mixture was dissolved in 100 ml of distilled water.
Tetrazolium test for hydroxamate siderophores [2]
To a pinch of tetrazolium salt, two drops of 2N NaOH and 0.1 ml of the culture filtrate were added. Then the appearance of a deep red colour which was an indication of hydroxymate type of siderophore was examined.
Estimation of Total cell protein
Total cell proteins were estimated by Lowry method [5]. An aliquot (0.5 ml) of the bacterial suspension was mixed with 0.5 ml of 1 N NaOH and kept in boiling water for 10 min. After cooling, 5 ml of copper carbonate reagent (50 ml of 2% sodium carbonate, 1 ml of 0.5 % copper sulphate and 1 ml of 1% sodium potassium tartarate) were added. The solution was allowed to stand at room temperature for 10 min. Then 0.5 ml of diluted (1:1 dilution with water) Folin Phenol reagent was added with vigorous shaking. After 30 min. the absorbance of the coloured solution was read at 660 nm against reagent blank. Bovine serum albumin was used as standard.
Estimation of Hydroxymate type siderophore [6]
The culture was grown in succinate minimal medium for 48 h at 120 rpm. An aliquot of 2ml of culture filtrate was mixed with 2 ml of 3M H2SO4 and hydrolysed by autoclaving it for 4h. After cooling, the hydrolysed solution was transferred into a 50 ml volumetric flask and 7 ml of 2 M sodium acetate solution was added. Then 2 ml of sulfanilamide solution (1% in 30% acetic acid) and 2 ml of iodine solution (0.65% in 1% KI solution) were added. The mixture was swirled and allowed to react for 5 min. The excess iodine was removed by the addition of 2 ml of sodium arsenite solution (1.5%), and 2 ml of N-(1-Naphthyl) ethylene diamine (NNEDA) solution (0.05%). The reaction mixture was left at room temperature for 30 min and made up to 50 ml with distilled water. The absorbance of the coloured solution was read at 543 nm. Hydroxylamine hydrochloride was used as standard.
RESULTS AND DISCUSSION
Siderophore Production
All the fluorescent pseudomonads isolate produced siderophores as evidenced by CAS assay, FeCl3 test and Spectrophotometric assay.
CAS assay
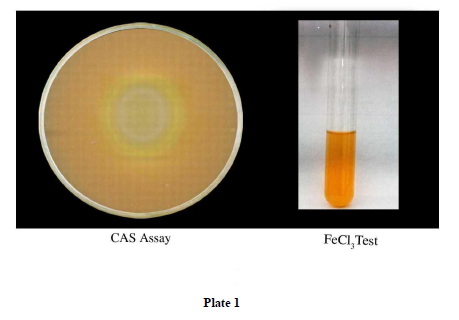
The secretion of siderophores was surveyed by CAS assay. The isolates were tested positive for CAS assay (Plate 1)
CAS is a method that can be used to detect the mobilization of iron. It is a universal test for detection and determination of siderophores, as even 0.02 μm of siderophores can be determined [7]. Alexander and Zeeberer [1] demonstrated that CAS agar effectively differentiated bacteria that were able to excrete large amounts of siderophore. The Fe (III) gives the agar a rich blue color and concentration of siderophores excreted by iron starved organisms results in a color change to orange [8].
FeCl3 Test
The formation of purple colour on addition of 1-5ml of 2% Fecl3 indicated the presence of siderophores. All the 22 isolates were detected to be Fecl3 test positive (Plate1)
Spectrophotometric assay
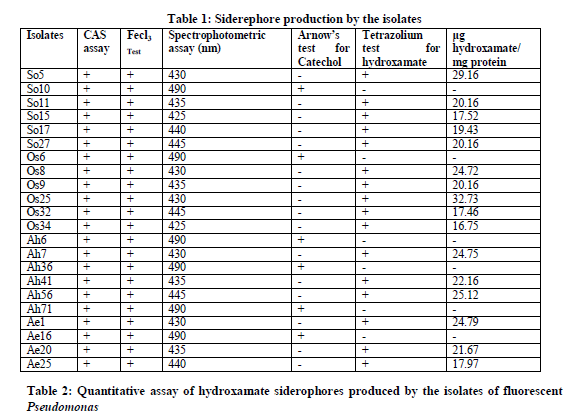
Spectrophotometric assay was performed to determine the nature of siderophores produced by the isolates. Hydroxamate, Catecholate and carboxylate nature of siderophores was ascertained by examining the absorption maxima in Hitachi UV-Vis 160 A spectrophotometer. The values of the maximum absorption peaks of the spectrophotometric assay of the isolates were documented in Table: 1.
The maximum absorption peaks between 420-450nm on addition of 1 ml of 2% Fecl3 to 1 ml of cell-free culture filtrate indicated the presence of hydroxamates. Among the 22 isolates, 16 isolates showed a peak between 420-450 nm and were identified as hydroxamate siderophores. A maximum absorption peak at 490 nm indicated the presence of catecholate siderophores which were observed in the remaining 6 isolates and were thus identified as catecholate type.
Determination of the chemical nature of the siderophores was also done by Arnow’s test and Tetrazolium test.
Arnow’s Test for catecholate siderophores
Catecholate siderophores on reaction, in sucession with nitrous acid, molybdate and alkali, yield a pink chromogen that absorbs maximally at 515 nm. Of the 22 isolates which showed the abilty to produce siderophores, 6 isolates were shown to produce catecholate type of siderophores (Table 1).
Tetrazolium test for hydroxamate siderophores
This test is based on the capacity of hydroxamic acids to reduce tetrazolium salts by hydrolysis of hydroxamate group in the presence of strong alkai [7]. Instant appearance of deep red colour on addition of tetrazolium salt and NaOH to the test sample indicated the presence of hydroxamate type of siderophores. Apart from the 6 isolates which have the ability to produce catecholate sideroohores, the remaining 16 isolates showed the presence of hydroxamate siderophores (Table 1).
Quantitative assay of hydroxamate siderophores
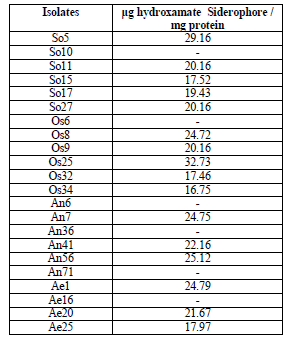
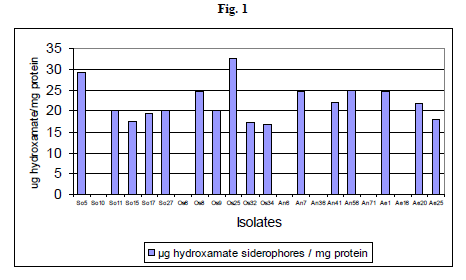
Hydroxamate type siderophore production was assayed quantitatively and it was found that different isolates produced varying amounts of siderophores. Among the hydroxamate siderophore producing isolates, six isolates produced high levels of siderophores that ranged between 32.73 to 24.12 μg hydroxymate / mg protein. The isolate P. fluorescens Os25 was found to produce highest quantity of siderophore, which was followed by P. fluorescens So5. The isolate P. fluorescens So5 produced 29.16 μg hydroxymate / mg protein. The isolates P. fluorescens Os32 and P. fluorescens Os34 produced less quantity of siderophores amounting to 17.46 μg hydroxymate / mg protein and 16.75 μg hydroxymate / mg protein respectively (Table 2 and Fig 1).
Growth and pyoverdine production kinetics of Pseudomonas aeruginosa 7NSK2 under uncontrolled pH condition were studied in an experimental fermentor by Fallahzadeh et al. [9] and they found that the yield of the pyoverdine production was 11.44 µMs pyoverdine / g glucose. Siderophore production of strains 35Q and 113 on 8-HQ+KB medium, was investigated by Fallahzadeh-Mamaghani et al. [10] and they found that siderophore production by both 35Q was significantly higher with final concentration of siderophore produced being 381.7 µM. and siderophore production by strain 113 was lower because it was not able to grow on KB medium with high concentrations of 8-HQ.
Most known siderophores can be grouped into hydroxamate and catecholate type structures and has different affinities for ferric iron. Metzanke [11] reported that Catecholate type siderophores generally have higher formation constants with ferric iron and the stability of these iron complexes is highly pH dependent. Neilands [12] observed that the hydroxamate complex is much more stable and hence potentially more significant in the rhizosphere.
Kloepper et al. [13] reported that siderophore production by fluorescent pseudomonads is partially responsible for enhanced plant growth. Leong [14] observed that the siderophores synthesized by fluorescent pseudomonads reduced the population of deleterious microorganisms in rhizoplane and in rhizosphere of plants, and made the roots healthier. Siderophore producing Pseudomonas putida induced better root development in potato, but not the mutant strains defective in the biosynthesis of siderophores [15, 16]. Siderophore mediated biological control has been reported in several instances.
Fluorescent pseudomonads are capable of producing hydroxymate and catecholate type of siderophores. In the present study 6 isolates were capable of producing catecholate type and 16 have the ability to produce hydroxamate type of siderophores.
CONCLUSION
Many experiments suggest that production of siderophores when iron is limited is responsible for the antagonism of the phytopathogens. The use of bioinoculants based on fluorescent pseudomonads appears as a worthy approach for disease management. Also Application of siderophore producing microorganisms in roots would significantly amend the iron insufficiency in plants.
References:
- Alexander, D.B. and Zuberer, D.A. (1991). Use of chrome azurol S reagents to evaluate siderophore production by rhizosphere bacteria. Biology and Fertility of Soils. 12 : 39-45.
- Snow, G. A. (1954) . Mycobactin. A growth factor for Mycobacterium jhnei. II. Degradation and identification of fragments. J. Chem. Soc. 10 : 2588-2596.
- Jalal, M. A. F. and Vander Helm, D. (1990). Isolation and spectroscopic identification of fungal siderophores. In: Handbook of microbial iron chelates. Winklemann, G. (ed.), Pegamon Press, Oxford, pp. 235-269.
- Arnow, L. E. (1937). Colorimetric determination of the components of 3,4-dihydroxyphenylanine tyrosine mixtures. J. Biol. Chem. 118 : 531-537.
- Lowry, O.H., Rosenbrough, N.J., Farr, A.L. and Randall, R.J. (1951). Protein measurement with the folin phenol reagent. J. Biol. Chem. 193: 265-275.
- Csaky, T. Z. (1948). On the estimation of bound hydroxamines in biological materials. Acta Chem. 2 : 450-454.
- Dave, B. P., Kene Anshuman and Puja Hajela. (2006). Siderophores of Halophilia archaea and their chemical characterization. Idian Journal of Experimental Biology. 44 : 340-344.
- Schwyn, B. and Neilands, J. B. (1987). Universal Chemical Assay for the Detection and Determination of Siderophores. Analytical Biochemistry. 160 : 47-56.
- Fallahzadeh, V., Ahmadzadeh, M. and Sharifi, R. (2010). Growth and pyoverdine production kinetics of Pseudomonas aeruginosa 7NSK2 in an experimental fermentor. Journal of Agricultural Technology .6(1) : 107-115.
- Fallahzadeh-Mamaghani, V., Ahmadzadeh, M. and Sharifi, R. (2009). Screening systemic resistance-inducing fluorescent pseudomonads for control of bacterial blight of cotton caused by Xanthomonas campestris pv.malvacearum. Journal of Plant Pathology. 91(3) : 663-670.
- Metzanke, B. F. (1987). Mossbauer spectroscopy of microbial iron metabolism, p. 251-284. In G. Winkelmann, D. van der Hehn, and J. B. Neilands;(ed.), Iron transport in microbes, plants and animals. Verlagsgeselischaft mbH, Weinheim.
- Neilands, J. B. (1981). Microbial iron compounds. Annu. Rev. Biochem. 50 : 715-731.
- Kloepper, J. W., Leong, J. Teintze, M., and Schroth, M. N. (1980). Enhanced plant growth by siderophores produced by plant growth promoting rhizobacteria. Nature. 286 : 885-886.
- Leong, J. (1986). Siderophores: Their biochemistry and possible role in the biocontrol of plant pathogens. Ann. Rev. Phytopathol. 24 : 187-209.
- Baker, R., Elad, Y. and Sneh, B. (1986). Physical, biological and host factors in iron competition in soils. In : Swinburne, T. R. (ed). Iron siderophores and plant diseases. Plenum Press, New York, pp 77–84.
- Schippers, B., Geels, F. P., Bakker, P. A. H. M., Bakker, A. W., and Weisbeek, P. J. (1986). Methods of studying plant growth promoting pseudomonads-problems and progress. NATO, Adv. Res. Workshop: Iron, siderophores and plant disease. Ashford, Kent, U. K .
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License