IJCRR - 9(21), November, 2017
Pages: 01-12
Date of Publication: 01-Jan-0001
Print Article
Download XML Download PDF
Lignocellulose Degrading Enzymes from Fungi and Their Industrial Applications
Author: Perinbam Kantharaj, Bharath Boobalan, Seeni Sooriamuthu, Ravikumar Mani
Category: General Sciences
Abstract:The rich diversity of fungi and diverse range of enzymes produced by them together make researchers to exploit their potential for various industrial applications. Few of the fungal enzymes have already been harnessed and many other are to be explored and brought into use. Recent studies suggested that the lignin degrading fungi can be used in the bioremediation of aromatic hydrocarbons including dioxins, dibenzofuran, aromatic dyes, etc. Employing fungal enzymes for the treatment of pollutants has gained attraction recent days for their selectivity, specificity and eco-friendly nature. Of these enzymes, peroxidases (lignin peroxidase and manganese peroxidase) and laccases are the two major classes of enzymes involved in biodegradation of lignin and recalcitrant xenobiotics. In addition, cellulase and hemicellulase were found to play a role in the management of lignocellulosic wastes. The present review gives a detailed account on the various lignocelluloses degrading enzymes, their fungal sources and their industrial applications. \? \? \? \?
Keywords: Peroxidases, Lignocelluloses degradation, Xenobiotics, Fungal sources, Industrial applications
Full Text:
INTRODUCTION
Lignocelluloses are the main structural component of all plants and most of the industries including forestry, agriculture, food, pulp and paper are producing large amount of lignocellulosic wastes1-4. Most of the agricultural residues are rich in non-edible lignocelluloses and serve as renewable sources for the production of various value added products including biofuel which can act as the replacement for the fossil fuels5. Alternative fuels of petroleum solve many of the current social problems and concerns, from air pollution and global warming to other environmental improvements and sustainability issues6 In order to exploit the uses of lignocellulosic biomass, several physical and chemical processes have been developed for the separation of cellulose, hemicellulose, and lignin from them. The separation processes include chemical viz. alkali, acid, ammonia and lime and microwave pre-treatments (physical)7. The commercial pre-treatment process carries respective drawbacks including decrease in the quality of the polymers, release of by-products that inhibit the fermentation of resulting sugars, etc.
In order to overcome these drawbacks, biocatalysts (enzymes) can be used to improve the superiority of the pretreatment process8,9. In turn, enzymes produced by wood decaying fungi serve as an important factor for the conversion of organic debris into humus and helps in the carbon and nitrogen cycling. The lignocellulytic activity of the fungi is also facilitated with the help of extracellular enzymes, such as cellulases, hemicellulases, MnP (Manganese peroxidase), LiP (Lignin Peroxidase) and Lac (Laccase). These enzymes can be used in the management of environmental pollutants such as textile effluents, pulp effluents, organochloride agrochemicals and crude oil residues10,11. The filamentous fungi are rich in the production of extracellular lignocellulolytic enzymes, when compared to bacteria and yeast12. Since, today's world demand for more constant, active and specific enzymes, wood decaying fungi serve as an ideal candidate for the management of lignocellulosic wastes. In order to exploit the uses of lignocellulosic biomass, enzymes produced by wood decaying fungi can be used as an important factor for the conversion of organic debris into humus and helps in production of value added products. Even though many fungal species are involved in the biodegradation of pollutants including xenobiotics, it is essential to investigate their sources, diversity and mode of action. The present review will aid to acquire knowledge of different lignocellulosic enzymes, the fungal strains responsible for their production and their industrial applications.
Plant cell wall
Plant cell wall is a multifaceted composite of polysaccharides, aromatic compounds, proteins, etc. The plant cell wall consists of three important lingocellulosic components which include cellulose, hemicelluloses and lignin. In a plant, the lingo-cellulose materials comprise 30-50% of cellulose, 15-30% hemicelluloses and 15-35% of non-carbohydrate aromatic polymers the composition vary based on the species, morphology and age of the plant13,14. The secondary cell wall is synthesized and differentiated by cellulose microfibrils with superior crystallinity and altered hemi-cellulose content15. The large quantity of lingo-cellulosic materials present in cell wall make them the abundantly present, potentially inexpensive and easily available natural resources for the production of biofuels and high value compounds16. The use of lignocellulosic materials primarily involves the separation of the polymeric compounds into cellulose and hemicelluloses. In the absence of potential enzymes, the natural degradation of such lignocelluloses is very slow: however, microorganisms in the soil are capable to degrading the compounds and converting them into sugars at faster rate. Microorganisms capable of growing on lignocellulosic materials produce a wide range of enzymes that could be of scientific and industrial importance. Moreover, the alcohols produced by the utilization of ligocellulosic wastes could be utilized as a biofuel. Also, chemicals like vanillin, xylitol, and furfural obtained from lingocellulosic wastes can be used in industrial products including herbicides, pharmaceuticals, and household products17,18.
Wood decaying fungi
The omnipresent fungi are the extensive producers of hydrolyzing enzymes which are responsible for the degradation of carbohydrate present in dead plant biomass19,20. Generally, fungi require favorable temperatures (32o - 90o F), nutrients and sufficient source of oxygen for them to survive and multiply. Since forests represent the major biome of the earth, fungi inhabiting the forests are able to degrade and mineralize the major chunk of ligno-cellulosic substrates. Fungi can be differentiated into different classes based on their distinct spore structures including Ascomycetes, Basidiomycetes and Deuteromycetes21. The wood decaying fungi use both enzymatic and non-enzymatic system for the degradation and complete decomposition of wood. In the wood decay process, wood turns discolored and loses weight, strength and density by the action of fungi. Most of the fungi involved in degradation of lignin and hemicelluloses fall into three broader groups namely, brown-rot, white-rot and soft-rot fungi22.
Brown-rot fungi
The brown-rot fungi generally reduce the strength of wood upto 75% by decomposing the cell wall polymers such as cellulose and hemicellulose leaving behind the lignin23. Brown rot fungi make the wood fragile, dry and crumble into cubes due to the formation of longitudinal and transverse cracks24. The brown rot fungi dry out, makes wood into to powder when crushed and it is characterized by reddish brown color and dry, crumbly and brittle consistency . Brown rot is often referred as "dry rot". Poria incrassate is one of the water conducting brown rot fungi having specific rhizomorphs based on root-like water-conducting tubes to transport water from the soil to the wood and can be decayed by the fungus. Once the brown rot fungus infected, it can rapidly multiply from side to side building and destroying large areas of floor covering and walls in one or two years. Examples of such wood decaying brown-rot fungi include Gloeophyllum trabeum, Fomitopsis lilacino-gilva, Laetiporus portentosus, Postia placenta and Serpula lacrymans24,25. In contrast, the numerous enzymes secreted by brown-rot and white-rot fungi enhance the wood degradation26.
White-rot fungi
White-rot fungi belong to the family, Basidiomycetes which gradually utilize all major cell wall components such as carbohydrates, lignin and aromatic compounds27,28. Ceriporiopsis subvermispora and Phlebia radiata are the two best studied white rot fungi to elicit white-rot decay29,30. The white rot fungi produce three classes of extracellular ligninolytic enzymes: laccase, lignin peroxidase and manganese peroxidase that produce H2O2 needed for peroxidase activities. The white rot fungi Rigidoporous lignosus is known to produce two oxidative enzymes such as MnP and laccase which is capable breaking down the lignin in a synergistic system31. The mixed cultures of white-rot fungi are also found to improve laccase production32. Dichornitus sqiualenis appeared to delignify early wood cells, whereas, Phellinuis pini delignifies latewood cells effectively. Otjen33 observed decay patterns in oak caused by Inonotits diyophillis which demonstrated that the fungus has a preference of early wood fibers and parenchyma cells but not latewood fibers.
Soft rot fungi
Soft rot fungi otherwise referred to as micro fungi were characterized by cavity formation in the secondary walls of the wood cells34. Generally, soft rot fungi utilize cellulose and hemicellulose. Soft rot fungi degrade wood at slower rate compared to brown rot fungi and white rot fungi. In general they are found in wet floor boards, rotting window frames and fence posts. Some of these fungi are common decomposers of cellulose in soil and they are the least specialized wood decaying fungi.
Enzymes involved in lignocellulose degradation
Laccases and peroxidases are major lignolytic enzymes involved in enzymatic lignin degradation35,13. In addition, cellulose, hemicellulase and pectinase also play role in lignocellulosic waste degradation. Particular significance is attached to fungi producing the lignocellulosic enzymes (Table. 1) and their role in the process will be discussed.
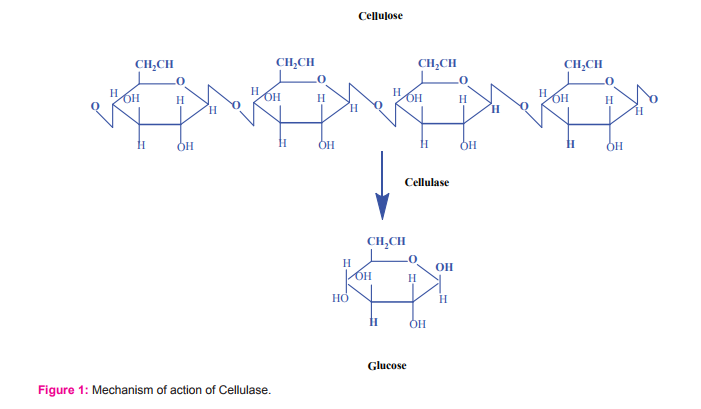
Cellulases
Cellulase hydrolyses the glycoside bond present between the glucose residues in the organic polymer cellulose (Fig.1). Cellulose can be hydrolyzed by β-1,4-endoglucanases, exoglucanases or 1,4-β-cellobiosidase, and β-glucosidase 36-38. Immanuel39reported cellulase production by Aspergillus niger and Aspergillus fumigates and optimized the parameters including pH, inoculums size, temperature, presence of inducers, etc. Trichoderma reesei is identified as the efficient cellulase producer by many researchers to degrade the cellulose40-42. Elyas43 and Dubrovskaya44 have isolated β-glycosidase enzyme from marine derived fungi such as Aspergillus sp. and Penicillum canescens. The amount of β-glucosidase in the Trichoderma cellulase system is reported to be lower than that needed for the efficient saccharification of lingocelluloses45. In a recent study, the cellulose produced by the Aspergillus sp. was used for the enzymatic saccharification of lignocellulosic agrowaste7. In addition, the production of cellulase has been widely studied in P. chrysosporium, Sclerotium rolfsii, Aspergillus sp., Penicillium sp., Schizophyllum sp. and Trichoderma sp.46-48.
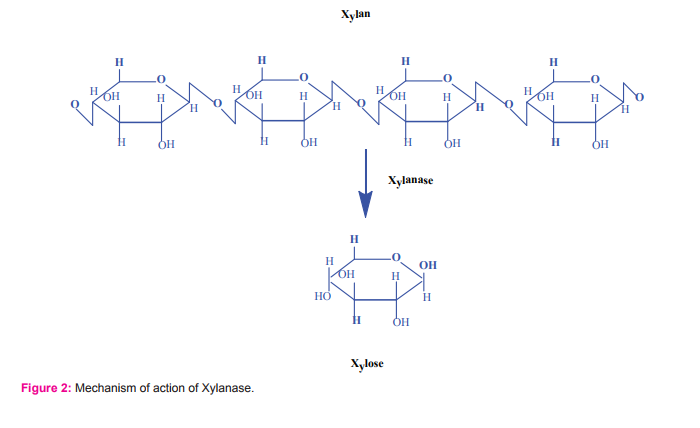
Hemicellulase
Hemicellulase such as xylanase are hydrolyses the xylan(Fig. 2) are extensively studied and applied on industrial scale with higher pulp brightness resulting in a lower chemical input49. In a recent study, a cold active xylanase was isolated from a marine fungus, Cladosporium sp50. In addition, the xylanase and endo-xylanase production has been widely studied in fungi such as Penicillium thomii51, P. pinophilum52,53, A. niger54and Ceratocystis paradoxa55. From an industrial point of view, an alkaline xylanase producing fungi, A. niger56and P. canescens57were isolated from marine sources.
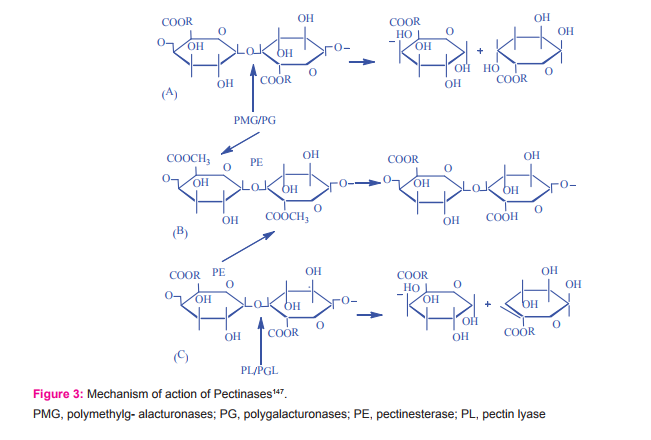
Pectinase
The pectinolytic enzymes are produced by both plants and microorganisms. In plants, the pectinases are concerned with fruit ripening and softening whereas, pectinase produced by microorganisms helps in the degradation of the dead vegetable biomass for their utilization in soil fertilizer and nutrient recycling58,59. The pecinases degrade the pectins (Fig. 3) via depolymerization and de-esterification reactions60. Pectinase production has been studied in the following group of microscopic fungal species: Aspergillus, Penicillium, Colletotrichum, Sclerotina, Fusarium, Trichoderma, Verticulum, Sclerotium, Geotrichum61-66. Among them A. niger was found to be a good producer of commercial67,68. Many industrial firms are involved in the commercial production of pectinases used in protoplast isolation whose purity and activity vary from one source to another. Pectinase production has also been studied in phytopathogenic Ascomycetes including, Neurospora crassa, Thermoascus aurantiacus, Rhizoctonia sp69,70 yeast like Saccharomyces cerevisiae71and Zygomycetes such as Mucour sp. and Rhizopus sp.72,73.
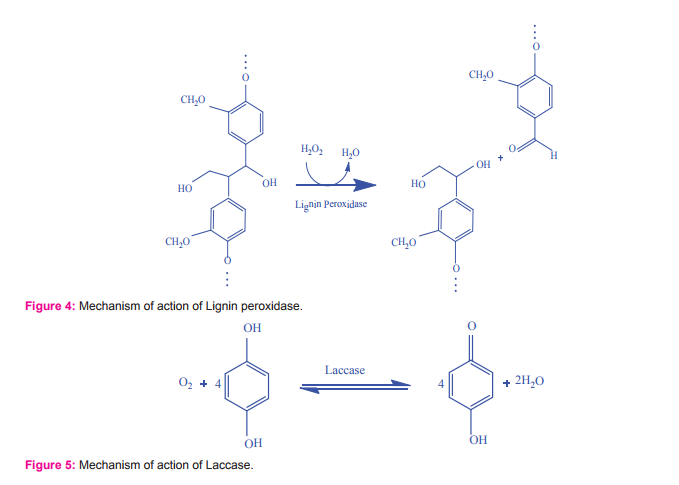
Lignin Peroxidase
Lignin peroxidases are the heme glycoprotein that plays a vital role in lignin degradation (Fig. 4), which cleaves C-C bonds and oxidizes benzyl alcohols to aldehydes or ketones74,75. Lignin peroxidases act on both phenolic (e.g. syringic acid, guaiacol, catechol, vanillyl alcohol, acteosyringone) and non-phenolic lignin substrates25. Mostly, basidiomycetes are shown to produce efficient lignin peroxidases76,25. Extracellular lignolytic enzymes are prominently produced by P. chrysosporium and P. radiata77whereas Coriolus tersicolor, are capable of producing intracellular lignolytic enzymes78. Researchers have studied the lignin peoxidase producing ability of different fungi including P. chrysosporium79, T. versicolor80, Pleurotus ostreatus81, Panus sp., P. coccineus, Perenniporia medullapanis, and P. sanguineus82.
Manganese peroxidase
Manganese peroxidase degrades the lignin mainly by attacking phenolic lignin component83. In the presence of H2O2, manganese peroxidase oxidizes the phenolic structures by converting Mn2+ to Mn3+. Oxalate and malonate are the mediators that produce carbon centered radicals, peroxyl radicals and superoxide radicals which improves the effective lignin-degrading system83,25. Manganese peroxidase is an essential component to certain basidiomycetes and some wood decaying white-rot fungi, which secrete manganese peroxidase in several forms into their environment. Among the basidiomycetes, Agaricus bisporus84, Lenzites betulinus85, Panus tigrinus86and Nematoloma frowardii87are identified to produce more stable manganese peroxidases. Järvinen88 have studied MnP production on selected lignin degrading organisms P. chrysosporium, Physisporinus rivulosus, P. radiata and Bjerkandera sp. and found P. chrysosporium as best manganese peroxidase producer. Bonugli santos89 isolated marine fungi, Mucor racemosus which possess the ability to produce salt tolerant manganese peroxidase.
Laccase
Laccases are the copper containing polyphenol oxidases which enable degradation of phenolic compounds and also reduce molecular oxygen to water (Fig. 5)90-92. Laccases oxidize the phenolic units in lignin to phenoxy radicals, which can lead to aryl-C cleavage93. Laccase can also oxidize non-phenolic substrates in the presence of certain auxiliary substrates94. A large variety of fungal strains isolated from several sea grasses, algae and decaying wood samples possess the ability to produce laccase enzyme. Atalla95 have isolated Trematosphaeria mangrovei from mangrove ecosystem which produces laccase enzyme at significant quantity. A thermo stable, metal-tolerant laccase is reportedly produced by marine-derived fungi, Cerrena unicolor96. Various researchers have isolated laccase producing fungi from different sources including Trichoderma harzianum97, Trichoderma atroviride98 and Trichoderma longibrachiatum99, Trametes versicolor100, Lentinus tigrinus101, Trametes pubescens102, Cyathus bulleri103, Paecilomyces sp.104, P. chrysosporium105, Lentines edodes106 and Pleurotus ostreatus107,81, Ganoderma lucidum91, Alternaria tenuissima108 and Trichoderma sp.92.
Applications of lignocellulytic enzymes
Lignocellulytic enzymes are industrially very useful and the fungal cellulases are having emerging applications in various industries like fruit juice processing, ruminant nutrition for improving digestibility and de-inking of paper109,110. A cellulase produced by Aspergillus sp. was used as refining aid for cotton comber pulp, and was changed into value added security paper111. The cellulase obtained from fungal sources also plays a key role in the preparation of household detergents and are also used in textile industry for bio-polishing of fabrics, stonewashing of denims112. The cellulase is also used in the animal feeds for increasing the nutritional quality, to develop digestibility113-115. Fungal hemicellulases are used in the production of chemical pulps and improving pulp beat ability of unbleached pulps116-117.
Fungal pectinases are being used in other industries such as textiles, plant fiber processing, tea, coffee, oil extraction, treatment of industrial wastewater, paper making, etc.119-120. Among the fungal sources, A. niger produces commercial pectinases which are used in the fruit juice and wine making industries. Pectinase accounts for 7.5% in the global enzyme market costing approximately 75 million USD72. The major applications of the pectinase enzymes are found in vegetable and fruit processing, where the removal of undesired pectin during extraction and clarification of fruit juice, wine, and cider is carried out.
There is an enormous interest in wood decaying fungi for large scale biodegradation applications due to their ability to produce large amount of extracellular lignocellulolytic enzymes28. Mtui and Masalu121 have isolated a lignocellulolytic fungus, Laetiporus sulphureus, from mangrove forest having the ability to degrade cellulose, hemicellulose and lignin presented in the mangrove litter. Immobilized enzymes are employed in the pharmaceutical, food and chemical industries 122. Immobilization also facilitates the efficient recovery and reuse of costly enzymes, and enables their use in continuous, fixed-bed operation123. The enzyme produced by the fungi was also employed for the detoxification of aromatic pollutants like agrochemicals and industrial effluents. The lignolytic white rot fungi have found their potential applications in the fields such as decolorization of industrial dyes, bleaching of pulp from textiles and paper, and degradation of organo pollutants, etc.28,124. The salt tolerant lignin degrading enzymes from fungi can be used for the effective bioremediation of environment pollutants125. The MnP finds their major applications in biomechanical pulping, dye decolorization, biorefineries, bioremediation and pulp bleaching 126,127. In modern sensitive studies involving plant protoplast fusion and gene transfer processes, purified cellulases and pectinasers find immense use and Japanese are the pioneers in this field.
Sahadevan128 reported lignin-degrading enzymes, LiP, MnP and Laccase from MVI.2011 an alkalophilic fungus to afford an appropriate biological substitute to treat highly alkaline effluents like pulp, paper industry and waste water. Indira Priyadarsini129described that the ability of fungi to produce laccase was linked with the effective decolorization of azo dyes which can be exploited for the screening of laccase producers. The fungal laccases are widely used in the industries such as food, textile, wood processing, pharmaceutical and chemical industries. In recent years, laccases are widely studied for textile industry in denim bleaching130,131. Another important application of laccase is the bioremediation of poisonous organic pollutants like chlorophenols and polycyclic aromatic hydrocarbons from the soil132,133. The stable laccase enzyme produced by A. tenuissima is being used in several bioprocesses, such as biopulping, biobleaching, bioremediation, food technological uses, and treatment of industrial waste water134-136.
Conclusion
Among the three groups Ascomycetes, Basidiomycetes and Deuteromycetes organisms producing lignocellulosic enzymes, Basidiomycetes group of fungi are considered as the promising candidates for the degradation lignocellulosic biomass. Even though many fungal species are involved in the biodegradation of pollutants, it is essential to augment the reactions by the development of new strains and employing microbial consortium or enzymatic cocktails for industrial applications. The enzyme production by the filamentous fungi are having biotechnological importance due to their applications in different fields including plant protoplast culture and protoplast fusion. The typical ecosystems present a veritable emporium of such organisms which are as yen poorly understood and commercially less exploited. When compared to cellulose and hemi-cellulose, lignin is found to be most difficult to degrade. For the hydrolysis of lignin, in addition to physical and chemical elements, addition of enzyme will be effective in terms of economic use as well as eco-friendly and sustainable use. The present review will aid to acquire knowledge of different lignocellulosic enzymes, the fungal strains responsible for their production and their industrial applications. Further studies are necessary to investigate the industrial applications of these enzymes for emerging production and innovation of new fungal strains.± ± ± ± ± ±
Acknowledgment
Authors also acknowledge the immense help received from the scholars whose articles are cited and included in refeences of this manuscript. The authors are also grateful to authors/ editors/ publishers of all the articles, journals and books from where the literature for this article has been reviewed and discussed.
Conflict of interest
There is no conflict of interest.
Table 1: List of fungi producing lingo-cellulolytic enzymes
|
ORGANISM
|
ENZYME PRODUCED
|
REFERENCES
|
|
A. niger, A. fumigates
|
Cellulase
|
Immanuel et al.39
|
|
T. reesei
|
Cellulase
|
Stricker et al.40; Kubicek et al.42
|
|
Aspergillus sp.
|
Cellulase
|
Elyas et al.43
|
|
P. canescens
|
Cellulase
|
Dubrovskaya et al.44
|
|
Aspergillus sp.
|
Cellulase
|
Bhavsar et al.7
|
|
P. chrysosporium
|
Cellulase
|
Saratale et al.48
|
|
Trichoderma viride
|
Cellulase
|
Iqbal et al.146
|
|
Cladosporium sp.
|
Xylanase
|
Del-Cid et al.50
|
|
P. thomii
|
Xylanase
|
Palaniswamy et al.51
|
|
P. pinophilum
|
Xylanase
|
Li et al.52; Lee et al.53
|
|
A. niger
|
Xylanase
|
Sharma et al. 54
|
|
C. paradoxa
|
Xylanase
|
Dekker and Richards55
|
|
A. niger
|
Xylanase
|
Raghukumar et al.56
|
|
P. canescens
|
Xylanase
|
Burtseva et al.57
|
|
A. niger
|
Pectinase
|
Sakai et al.58; Martens and Schaap68
|
|
N. crassa
|
Pectinase
|
Marcus et al.69
|
|
T. aurantiacus
|
Pectinase
|
Rombouts and Pilnik63
|
|
Rhizoctonia sp.
|
Pectinase
|
Martins et al.70
|
|
S.cerevisiae
|
Pectinase
|
Poondlaet al.71
|
|
Mucour sp.
|
Pectinase
|
Kashyap et al. 72
|
|
Rhizopus sp.
|
Pectinase
|
Kolarova and Augustin73
|
|
P. radiate
|
Lignin peroxidases
|
Lee et al.77
|
|
C. tersicolor
|
Lignin peroxidases
|
Lobarzewski 78
|
|
Schizophyllum commune
|
Lignin peroxidases
|
Asgher et al.137
|
|
P. chrysosporium
|
Lignin peroxidases
|
Zeng et al.138; Junnarkar et al.139
|
|
T. versicolor
|
Lignin peroxidases
|
Johansson et al.80; Asgher et al.140
|
|
P. ostreatus
|
Lignin peroxidases
|
Sivakami et al.81
|
|
P. sanguineus
|
Lignin peroxidases
|
Pointing et al.82
|
|
A. bisporus
|
Manganese peroxidase
|
Lankinen et al.84
|
|
L. betulinus
|
Manganese peroxidase
|
Hoshino et al.85
|
|
T. suaveolens
|
Manganese peroxidase
|
Knezevic et al.141
|
|
P. tigrinus
|
Manganese peroxidase
|
Lisov et al.86
|
|
Trametes villosa
|
Manganese peroxidase
|
Silva et al.142
|
|
N. frowardii
|
Manganese peroxidase
|
Hilden et al.87
|
|
P. chrysosporium
|
Manganese peroxidase
|
Järvinen et al.88
|
|
P. rivulosus
|
Manganese peroxidase
|
Hakala et al.143
|
|
P. radiate
|
Manganese peroxidase
|
Hilden et al.144
|
|
Bjerkandera sp.
|
Manganese peroxidase
|
Järvinen et al.88
|
|
M. racemosus
|
Manganese peroxidase
|
Bonugli santos89
|
|
T. mangrovei
|
Laccase
|
Atalla, et al.95
|
|
C.unicolor
|
Laccase
|
D'Souza-Ticlo et al.96
|
|
T. harzianum
|
Laccase
|
Holker et al.98
|
|
T. atroviride
|
Laccase
|
Velazques et al.99
|
|
T. longibrachiatum
|
Laccase
|
Kiiskinen et al.10
|
|
T. versicolor
|
Laccase
|
Han et al.100; Asgher et al.154
|
|
L. tigrinus
|
Laccase
|
Ferraroni et al.101
|
|
T. pubescens
|
Laccase
|
Shleev et al.102
|
|
C. bulleri
|
Laccase
|
Salony et al.103
|
|
Paecilomyces sp.
|
Laccase
|
Liang et al.104
|
|
P. chrysosporium
|
Laccase
|
Viswanath et al.105
|
|
L. edodes
|
Laccase
|
Shanmugam et al.106
|
|
P. ostreatus
|
Laccase
|
Patel et al.107; Sivakami et al.81
|
|
G. lucidum
|
Laccase
|
Li et al.91
|
|
T. suaveolens
|
Laccase
|
Knezevic et al.141
|
|
A. tenuissima
|
Laccase
|
Abd El Aty et al.108
|
|
Trichoderma sp.
|
Laccase
|
Divya et al.92
|
References:
- Kim S, Dale BE. Global potential bioethanol production from wasted crops and crop residues. Biomass Bioenergy 2004; 26: 361-375.
- Wen Z, Liao W, Chen S. Hydrolysis of animal manure lignocellulosics for reducing sugar production. Bioresource Technology 2004; 91: 31-39.
- Champagne P. Feasibility of producing bioethanol from waste residues: A Canadian perspective Feasibility of producing bio-ethanol from waste residues in Canada. Resource Conservation and Recycling 2007; 50: 211- 230.
- Sahadevan LDM, Misra CS, Thankamani V. Ligninolytic enzymes for application in treatment of effluent from pulp and paper industries. Universal Journal of Environmental Research and Technology 2013; 3: 14-26.
- Brustarr M, Bakenhus M. Economical, high efficiency engine technologies for alcohol fuels. (http://wwwepagov/otaq/presentations/ epa-fev-isaf-no55pdf) 2008.
- Kanimozhi S, Perinbam K. Molecular studies on lipase of Pseudomonas fluorescensLP1 and its application in biodiesel production. Chembiosis 2012; 3(1): 33-38.
- Bhavsar NH, Raol BV, Amin SS, Raol GG. Production, Optimization and Characterization of Fungal Cellulase for Enzymatic Saccharification of Lignocellosic Agro-waste. International Journal of Current Microbiology and Applied Sciences 2105; 4(3): 30-46
- Lynd LR, Laser MS, Bransby D, Dale BE, Davison B, Hamilton R, Himmel M, Keller M, McMillan JD, Sheehan J, Wyman CE. How biotech can transform biofuels. Nature Biotechnology 2008; 26(2): 169-172.
- Brijwani K, Vadlani PV. Cellulolytic Enzymes Production via Solid-State Fermentation: Effect of Pretreatment Methods on Physicochemical Characteristics of Substrate. Enzyme Research 2011; 2(1): 120-128.
- Kiiskinen LL, Rättö M, Kruus K. Screening for novel laccase-producing microbes, Journal of Applied Microbiology 2004; 97(3): 640-646.
- Mtui G, Nakamura Y. Lignin-degrading enzymes from mycelial cultures of basidiomycetes fungi isolated in Tanzania. Journal of Chemical Engineering of Japan 2004; 37(1): 113-118.
- Haltrich D, Nidetzky B, Kulbe KD, Steiner W, Zupaneie S. Production of fungal xylanases. Bioresource Technology 1996; 58: 137-161.
- Perez J, Munoz-Durado J, de la Rubia T, Martinez J. Biodegradation and biological treatment of cellulose, hemicellulose and lignin : an overview. International Microbiology 2002; 5: 53-63.
- Menon V, Rao M. Trends in bioconversion of lignocellulose: biofuels, platform chemicals and biorefinery concept. Progress in Energy and Combustion Science 2012; 38: 522-550.
- Ding SY, Himmel ME. The maize primary cell wall microfibril: A new model derived from direct visualization. Journal of Agricultural and Food Chemistry 2006; 54(3): 597-606.
- Himmel ME, Ding SY, Johnson DK, Adney WS, Nimlos MR, Brady JW, Foust TD. Biomass recalcitrance: engineering plants and enzymes for biofuels production. Science 2007; 315: 804-807.
- Howard RL, Abotsi E, Jansen van Rensburg EL, Howard S. Lignocellulose biotechnology: issue of bioconversion and enzyme production. African Journal of Biotechnology 2003; 2(12): 602-619.
- Ragauskas AJ, Williams CK, Davison BH, Britovsek G, Cairney J, Eckert CA, Frederick WJ Jr, Hallett JP, Leak DJ, Liotta CL, Mielenz JR, Murphy R, Templer R, Tschaplinski T. The path forward for biofuels and biomaterials. Science 2006; 311: 484-487.
- Rabinovich ML, Melnik MS, Bolobova AV. Microbial Cellulases- A Review. Applied Biochemistry and Microbiology 2002; 38: 305-321.
- Sanchez C. Lignocellulosic residues: biodegradation and bioconversion by fungi. Biotechnology Advances 2009; 27: 185-194.
- Kohlmeyer J, Kohlmeyer E. Marine Mycology -The Higher fungi. Academic Press, New York 1979.
- Schwarze F. Wood decay under the microscope. Fungal Biology Reviews 2007; 21(4): 133-170.
- Yelle DJ, Ralph J, Lu FC, Hammel KE. Evidence for cleavage of lignin by a brown rot basidiomycete. Environmental Microbiology 2008; 10: 1844-1849.
- Martínez AT, Speranza M, Ruiz-Dueñas FJ, Ferreira P, Camarero S, Guillén F, et al. Biodegradation of lignocellulosics: microbiological, chemical and enzymatic aspects of fungal attack to lignin. International Microbiology 2005; 8:195-204.
- Wong DW. Structure and action mechanism of ligninolytic enzymes. Applied Biochemistry and Biotechnology 2009; 157: 174-209.
- Jensen KA, Houtman CJ, Ryan ZC, Hammel KE. Pathways for extracellular Fenton chemistry in the brown rot basidiomycete Gloeophyllum trabeum. Applied and Environmental Microbiology. 2001; 67(6): 2705-2711.
- Baldrian P. Enzymes of saprotrophic basidiomycetes In: Ecology of Saprotrophic Basidiomycetes (eds Boddy L, Frankland JC, Van West P), Elsevier, Amsterdam 2008; 19-42.
- Dashtban M, Schraft H, Syed TA, Qin W. Fungal biodegradation and enzymatic modification of lignin. International Journal of Biochemistry and Molecular Biology 2010; 1(1): 36-50.
- Ander P, Eriksson KE. Selective degradation of wood components by white-rot fungi. Physiol Plant 1977; 41: 239-248.
- Fackler K, Schwanninger M, Gradinger C, Hinterstoisser B, Messner K. Qualitative and quantitative changes of beech wood degraded by wood rotting Basidiomycetes monitored by Fourier transform infrared spectroscopic methods and multivariate data analysis. FEMS Microbiology Letters 2007; 271(2): 162-169.
- Galliano H, Gas G, Seris JL, Boudet AM. Lignin degradation by Rigidoporus lignosus involves synergistic action of two oxidizing enzymes: Mn peroxidase and laccase. Enzyme Microbial Technology 1991; 13:478-482.
- Velazquez-Cedeiio MA, Farnet AM, Ferre E. Variations of lignocellulosic activities in dual cultures of Pleurotus ostreatus and Trichoderma longibrachiatum on unsterilized wheat straw, Mycologia 2004; 96: 712-719.
- Otjen J, Blanchette RA. Patterns of decay caused by Inonotius dryophilus (Aphyllophorales: Hymenochaetaceae) a white-pocket rot of oaks Can. Journal of Botany 1982; 60: 2270-2279.
- Savory JG, Breakdown of timber by Ascomycetes and Fungi Imperfecti. Annals of Applied Biology 1954; 41: 336-347.
- ten Have R, Teunissen P. Oxidative mechanisms involved in lignin degradation by white rot fungi. Chemical Reviews 2001; 11: 3397-3414.
- de Vries R, Visser J. Aspergillus enzymes involved in degradation of plant cell wall polysaccharides, Microbiology and Molecular Biology Reviews 2001; 65(4): 497-522.
- Kim SJ, Lee CM, Han BR, Kim MY, Yeo YS, Yoon SH, Jun HK. Characterization of a gene encoding cellulase from uncultured soil bacteria. FEMS Microbiology Letters 2008; 282: 44-51.
- Vlasenko E, Schulein M, Cherry J and Xu F, Substrate specificity of family 5, 6, 7, 9, 12, and 45 endoglucanases. Bioresource Technology 2010; 101(7): 2405-2411.
- Immanuel G, Bhagavath C, Iyappa Raj P, Esakkiraj P, Palavesam A. Production and Partial purification of Cellulase by Aspergillus niger and A fumigates fermented in coir waste and sawdust. International Journal of Microbiology 2009; 3(1): 1-11.
- Stricker AR, Mach RL, de Graaff LH. Regulation of transcription of cellulases and hemicellulases encoding genes in Aspergillus niger and Hypocrea jecorina (Trichoderma reesei). Applied Microbiology and Biotechnology 2008; 78(2): 211-20.
- Martinez D, Berka RM, Henrissat B, Saloheimo M, Arvas M, Baker SE, Brettin TS et al. Genome sequencing and analysis of the biomass degrading fungus Trichoderma reesei (syn Hypocrea jecorina). Nature Biotechnology 2008; 26(5): 553-560.
- Ravikumar M, Bala Kumaran MD, Balashanmugam P. Production of Cellulase Enzyme by Trichoderma reesei Cef19 and its Application in the Production of Bio-ethanol. Pakistan Journal of Biological Sciences 2014; 17: 735-9.
- Elyas KK, Mathew A, Sukumaran RK, Ali PPM, Sapna K, Kumar SR, Mol KR. Production optimization and properties of beta glucosidases from a marine fungus Aspergillus-SA58. Nature Biotechnology 2010; 27: 347-351.
- Dubrovskaya YV, Sova VV, Slinkina NN, Anastyuk SD, Pivkin MV, Zvyagintseva TN. Extra cellular beta-D-glucosidase of the Penicillium canescens marine fungus. Applied Biochemistry and Microbiology 2013; 48: 401-408.
- Xiao ZZ, Zhang X, Gregg DJ, Saddler JN. Effects of sugar inhibition on cellulases and beta-glucosidase during enzymatic hydrolysis of softwood substrates. Applied Biochemistry and Biotechnology 2004; 113: 1115-1126.
- Fan LT, Gharpuray MM, Lee YH. In: Cellulose Hydrolysis Biotechnology Monographs. Springer, Berlin, 1987; 57.
- Rana S, Kaur M. Isolation and screening of cellulase producing microorganisms from degraded wood. International Journal of pharma and bio sciences 2012; 2(1): 10-15.
- Saratale GD, Kshirsagar SD, Sampange VT, Saratale RG, Oh SE, Govindwar SP, Oh MK. Cellulolytic enzymes production by utilizing agricultural wastes under solid state fermentation and its application for biohydrogen production. Applied Biochemistry and Biotechnology 2014; 174: 2801-2817.
- Saha BC. Hemicellulose bioconversion. Journal of Industrial Microbiology and Biotechnology 2003; 30: 279-291.
- Del-Cid A, Ubilla P, Ravanal MC, Medina E, Vaca I, Levicán G, Eyzaguirre J, Chavez R. Cold-active xylanase produced by fungi associated with Antarctic marine sponges. Applied Biochemistry and Biotechnology 2014; 172: 524-532.
- Palaniswany M, Pradeep BV, Sathya R, Angayarkanni J. Isolation, identification and screening of potential xylanolytic enzyme from little degrading fungi. African Journal of Biotechnology 2008; 7(11): 1978-1782.
- Li Y, Liu Z, Cui F, Xu Y, Zhao H. Production of xylanase from a newly isolated Penicillium sp ZH-30. World Journal of Microbiology and Biotechnology 2006; 23(6): 837-843.
- Lee J, Jang Y, Lee H, Lee S, Kim GH, Kim JJ. Screening for xylanase and β-xylosidase production from wood-inhabiting Penicillium strains for potential use in biotechnological applications. Holzforschung 2011; 66(2): 267-271.
- Sharma S, Vaid S, Bajaj BK. Screening of thermo-alkali stable fungal xylanases for potential industrial applications. Current Research in Microbiology and Biotechnology 2015; 3(1): 536-541
- Dekker RFH, Richards GN. Purification, properties and mode of action of hemicellulase I produced by Ceratocystis paradoxa, Carbohydrate Research 1975; 39: 97-114.
- Raghukumar C, Muraleedharan U, Gaud VR, Mishra R. Xylanases of marine fungi of potential use for biobleaching of paper pulp. Journal of Industrial Microbiology and Biotechnology 2004; 31: 433-441.
- Burtseva YV, Sova VV, Pivkin MV, Anastyuk SD, Gorbach VI, Zvyagintseva TN. Distribution of O-glycosylhydrolases in marine fungi of the sea of Japan and the sea of Okhotsk: characterization of exo-cellular N-acetyl-beta-D-glucosaminidase of the marine fungus Penicillium canescens. Applied Biochemistry and Biotechnology 2010; 46: 648-656.
- Sakai T. Degradation of Pectins, in Winkelmann G (Ed), Microbial Degradation of Natural Products, Weinheim 1992; VCH: 57-81
- Paloma ki T, Saarilahti HT. Isolation and Characterization of New C Terminal Substitution Mutation Affecting Secretion of Polygalactur-Onases in Erwinia Carotovora ssp Carotovora. FEBS Letters 1997; 400: 122-126.
- Wood WA, Kellogg ST. (Eds), Methods in Enzymology, Vol: Biomass, Part B: Lignin, Pectin, and Chitin, Academic Press, New York 1988; 161: 315-322.
- Bailey M. Effect of temperature on polygalacturonase production by Aspergillus niger, Enzyme Microbial Technology 1990; 12: 622-624.
- Bailey MJ, Pessa E. Strain and process for production of polygalacturonase, Enzyme Microbial Technology 1990; 12: 266-271.
- Rombouts FM, Pilnik W. Pectic enzymes in W Pilmir (Ed): Microbial Enzymes and Bioconversion, Academic Press, London 1990; 227-282.
- Channe PS, Shewal JG. Pectinase production by Sclerotium rosfsii: effect of culture conditions. Folia Microbiologica. 1995; 40: 111-117.
- Teixeira MFS, Lima Filho JL, Duran N. Carbon sources effect on pectinase production from Aspergillus japonicus 586. Brazilian Journal of Microbiology 2000; 31: 286-290.
- Maldonado MC, Cáceres S, Galli E, Navarro AR. Regulation of the production of polygalacturonase by Aspergillus niger. Folia Microbiologica 2002; 47: 409-412.
- Sakai T, Sakamoto T, Hallaert J, Vandamme EJ. Pectin, pectinase and protopectinase, production, properties, and applications. Advances in Applied Microbiology 1993; 39: 213-294.
- Martens-Uzunova ES, Schaap PJ. Assessment of the pectin degrading enzyme network of Aspergillus niger by functional genomics. Fungal Genetics and Biology 2009; 46(1): 170-179.
- Marcus L, Barash I, Sneh B, Koltin Y, Finker A. Purification and characterization of pectolytic enzymes produced by virulent and hypo virulent isolates of Rhizoctonia solani Kuhn. Physiological and Molecular Plant Pathology 1986; 29: 325-336.
- Martins ES, Silva D, Da Silva R, Gomes E. Solid state production of thermo stable pectinases from thermophilic Thermoascus aurantiacus. Process Biochemistry 2002; 37: 949-954.
- Rombouts FM, Pilnik W. Pectic enzymes, in W. Pilmir (Ed.): Microbial Enzymes and Bioconversion. Academic Press, London 1990; 227-282.
- Kashyap DR, Vohra PK, Chopra S, Tewari R. Applications of pectinases in the commercial sector: a review. Bioresource Technology 2001; 77: 215-227.
- Kolarova N, Augustín J. Production of polysaccharide hydrolases in the genus Rhizopus. Folia Microbiologica. 2001; 46: 223-226.
- Tuor U, Winterhalten K, Fiechter A. Enzymes of white-rot fungi involved in lignin degradation and ecological determinants for wood decay. Journal of Biotechnology 1995; 41: 1-17.
- Piontek K, Smith AT, Blodig W. Lignin peroxidase structure and function. Biochemical Society Transactions 2001; 29: 111-116.
- Abbas A, Koc H, Liu F, Tien M. Fungal degradation of wood:initial proteomic analysis of extracellular proteins of Phanerochaete chrysosporium grown on oak substrate. Current Genetics 2005; 47: 49-56.
- Lee S, Ha, JK, Kang, HS, McAllister T, Cheng KJ. Overview of energy metabolism, substrate utilization and fermentation characteristics of ruminal anaerobic fungi Korean. Journal of Animal Nutrition Feedstuffs 1997; 21: 295-314.
- Lobarzewski J. The Characteristics and Functions of the Peroxidases from Trametes Versicolor in Lignin Biotransformation. Journal of Biotechnology 1990; 13(2-3): 111-117.
- Renganathan V, Miki K, Gold MH. Multiple molecular forms of diarylpropane oxygenase, an HO2-requiring, lignin-degrading enzyme from Phanerochaete chrysosporium. Archives of Biochemistry and Biophysics 1985; 241: 304-314.
- Johansson T, Welinder KG, Nyman PO. Isozymes of lignin peroxidase and manganese (II) peroxidase from the white-rot basidiomycete Trametes versicolor II Partial sequences, peptide maps, and amino acid and carbohydrate compositions. Archives of Biochemistry and Biophysics 1993; 300: 57-62.
- Sivakami V, Ramachandran B, Srivathsan J, Kesavaperumal G, Benila Smily D, Mukesh Kumar J. Production and optimization of laccase and lignin peroxidase by newly isolated Pleurotus ostreatus LIG 19. Journal of Microbiology and Biotechnology Research 2012; 2(6): 875-881.
- Pointing SB, Pelling AL, Smith GJ, Hyde KD, Reddy CA. Screening of basidiomycetes and xylariaceous fungi for lignin peroxidase and laccase gene-specific sequences. Mycological Research 2005; 109: 115-124.
- Asgher M, Bhatti HN, Ashraf M, Legge RL. Recent developments in biodegradation of industrial pollutants by white rot fungi and their enzyme system. Biodegradation 2008; 19: 771-783.
- Lankinen VP, Bonnen AM, Anton LH, Wood DA, Kalkkinen N, Hatakka A, Thurston CF. Characteristics and N-terminal amino acid sequence of manganese peroxidase from solid substrate cultures of Agaricus bisporus. Applied Microbiology and Biotechnology 2001; 55: 170-176.
- Hoshino F, Kajino T, Sugiyama H, Asami O, Takahashi H. Thermally stable and hydrogen peroxide tolerant manganese peroxidase (MnP) from Lenzites betulinus. FEBS Letters 2002; 530: 249-252.
- Lisov AV, Leontievsky AA, Golovleva LA. Hybrid Mn-peroxidase from the ligninolytic fungus Panus tigrinus 8/18 Isolation, substrate specificity, and catalytic cycle. Biochemistry (Moscow) 2003; 68: 1027-1035.
- Hilden KS, Bortfeldt R, Hofrichter M, Hatakka A, Lundell TK. Molecular characterization of the basidiomycete isolate Nematoloma frowardii b19 and its manganese peroxidase places the fungus in the corticioid genus Phlebia. Microbiology 2008; 154: 2371-2379.
- Järvinen J, Taskila S, Isomäki R, Ojamo H. Screening of white-rot fungi manganese peroxidases: a comparison between the specific activities of the enzyme from different native producers. AMB Express 2012; 2: 62.
- Bonugli-Santos RC, Durrant LR, DaSilva M, Sette LD. Production of laccase, manganese peroxidase and lignin peroxidase by Brazilian marine-derived fungi. Enzyme Microbial Technology 2010; 46: 32-37.
- Arora DS, Sharma RK. Ligninolytic fungal laccases and their biotechnological applications. Applied Biochemistry and Biotechnology 2010; 160(6): 1760-1788.
- Li P, Wang H, Liu G, Li X, Yao J. The effect of carbon source succession on laccase activity in the co-culture process of Ganoderma lucidum and a yeast. Enzyme Microbial Technology 201; 48: 1-6.
- Divya LM, Prasanth GK, Sadasivan C. Isolation of a salt tolerant laccase secreting strain of Trichoderma sp NFCCI-2745 and optimization of culture conditions and assessing its effectiveness in treating saline phenolic effluents. Journal of Environmental Sciences 2013; 25(12): 2410-2416.
- Kawai S, Umezawa T, Higuchi. Degradation mechanisms of phenolic P-1 lignin substructure model compounds by laccase of Coriolus versicolor. Archieves of Biochemistry and Biophysics 1988; 262(1): 99-110.
- Call HP, Muncke I. History, overview and applications of mediated lignolytic systems, especially laccase-mediator systems (lignozyme (R)-process). Journal of Biotechnology 1997; 53: 163-202.
- Atalla M, Zeinab H, Kheiralla Eman R, Hamed Amani A, Youssry Abeer A, Abd El Aty. Screening of some marine-derived fungal isolates for lignin degrading enzymes (LDEs) production, Agriculture and Biology Journal of North America 2010; 1(4): 591-599.
- DSouza-Ticlo D, Sharma D, Raghukumar C. A thermostable metal-tolerant laccase with bioremediation potential from a marine-derived fungus. Marine Biotechnology 2009; 11: 725-737.
- Savoie JM, Mata G, Mamoun M. Variability in brown line formation and extracellular laccase production during interaction between white-rot basidiomycetes and Trichoderma harzianum biotype Th2. Mycologia 2001; 93: 243-248.
- Holker U, Dohse J, Hofer M. Extracellular laccases in ascomycetes Trichoderma atroviride and Trichoderma harzianum. Folia Microbiologica 2002; 47(4): 423-437.
- Velazquez-Cedeiio MA, Farnet AM, Ferre E. Variations of lignocellulosic activities in dual cultures of Pleurotus ostreatus and Trichoderma longibrachiatum on unsterilized wheat straw. Mycologia 2004; 96: 712-719.
- Han MJ, Choi HT, Song HG. Purification and Characterization of Laccase from the White Rot Fungus Trametes versicolor. Journal of Microbiology 2005; 43(6): 555-560.
- Ferraroni M, Myasoedova NM, Schmatchenko V, Leontievsky AA, Golovleva LA, Scozzafava A, Briganti F. Crystal structure of a blue laccase from Lentinus tigrinus: evidences for intermediates in the molecular oxygen reductive splitting by multicopper oxidases. BMC Structural Biology 2007; 7: 60.
- Shleev S, Nikitina O, Christenson A, Reimann CT, Yaropolov AI, Ruzgas T, Gorton L. Characterization of two new multiforms of Trametes pubescens laccase. Bioorganic Chemistry 2007; 35: 35.
- Salony, Mishra S, Bisaria VS. Production and characterization of laccase from Cyathus bulleri and its use in decolourization of recalcitrant textile dyes. Applied Microbiology and Biotechnology 2006; 71: 646-653.
- Liang ZQ, Han YF, Chu HL. A new thermo tolerant Paecilomyces species which produces laccase and a biform sporogenous structure. Fungal Diversity 2007; 27: 95-102.
- Viswanath B, Subhosh Chandra M, Pallavi H, Rajasekhar Reddy. Screening and assessment of laccase producing fungi isolated from different environmental samples. African Journal of Biotechnology 2008; 7: 1129-1133.
- Shanmugam S, Rajasekaran P, Joseph Thanikal V. Synthetic dye decolourization, textile dye and paper industrial effluent treatment using white rot fungi Lentines edodes. Journal of Desalination and Water Treatment 2009; 4: 143-147.
- Patel H, Akshaya G, Shilpa G. Effect Of Different Culture Conditions and Inducers on Production of Laccase by a Basidiomycete Fungal Isolate Pleurotus Ostreatus HP-1 under Solid State Fermentation. BioResources 2009; 4: 268-284.
- Abd El Aty AA, El-Shamy AR, Atalla SMM, El-Diwany AI, Hamed ER. Screening of Fungal Isolates for Laccase Enzyme Production from Marine Sources, Research Journal of Pharmaceutical. Biological and Chemical Sciences 2015; 6: 221-228.
- Demain AL, Newcomb M, David Wu JH. Cellulase, clostridia and ethanol. Microbiology and Molecular Biology Reviews 2005; 69: 124-154.
- Sakthivel M, Karthikeyan N, Jayaveny R, Palani P. Optimization of culture conditions for the production of extracellular cellulase from Corynebacterium lipophiloflavum. Journal of Ecobiotechnology 2010; 2(9): 6-13.
- Gupta C, Jain P, Kumar D, Dixit AK, Jain RK. Production of cellulase enzyme from isolated fungus and its application as efficient refining aid for production of security paper. International Journal of Applied Microbiology and Biotechnology 2015; 3: 11-19.
- Saraswati Bai, Ravi kumar M, Mukesh kumar DJ, Balashanmugam P, Bala kumaran MD, Kalaichelvan PT, Cellulase Production by Bacillus subtilis isolated from Cow Dung. Archives of Applied Science Research 2012; 4(1): 269-279.
- Tolan JS, Foody B. Cellulase from submerged fermentation In:Advances in Biochemical Engineering: Biotechnology Recent Progress in Bioconversion of Lignocellulosics (Tsao, GT, Ed), Springer Verlag, Berlin 1999; 65 : 41-67.
- Ibrahim ASS, El-diwany AI. Isolation and identification of new cellulases producing thermophilic bacteria from an Egyptian hot spring and some properties of the crude enzyme. Australian Journal of Basic and Applied Sciences 2007; 1(4): 473-478.
- Ali UF, Saad El-Dein HS. Production and Partial Purification of Cellulase Complex by Aspergillus niger and Aspergillus nidulans Grown on Water Hyacinth Blend. Journal of Applied Sciences Research 2008; 4: 875-891.
- Paice MG, Lurasek L. Removing hemicellulose from pulps by specific enzymic hydrolysis. Journal of Wood Chemistry and Technology 1984; 4: 187-198.
- Noe P, Chevalier J, Mora F, Comtat J. Action of xylanases on chemical pulp fibers. Part II: Enzymatic beating. Journal of Wood Chemistry and Technology 1986; 6: 167-184.
- Clark TA, Mc Donald AG, Senior DJ, Mayers PR. Biotechnology in pulp and paper Manufacture, (Krik, TKand Chang, HM eds) Butterworth-Heine Mann Press Batson 1990; 153-167.
- Viikari L, Tenakanen M, Suurnakki A. Biotechnology in the Pulp and Paper Industry in Rehm HJ (Ed) Biotechnology. VCH-Wiley 2001; 523-546.
- Reid I, Richard M. Purified Pectinase Lowers Cationic Demand in Peroxide-Bleached Mechanical Pulp. Enzyme Microbial Technology 2004; 34: 499-504.
- Mtui G, Masalu R. Extracellular enzymes from brown-rot fungus Laetiporus sulphureus isolated from mangrove forests of coastal Tanzania. Academic Journals 2008; 3(4): 154-161.
- Kanimozhi S, Perinbam K. Immobilization of Lipase from Pseudomonassp.Lp1 by different techniques. Biotechnology 2011; 5(3): 1-4.
- Kanimozhi S, Perinbam K. Synthesis of amino-silane modified superparamagnetic Fe3O4 nanoparticles and its application in immobilization of lipase from Pseudomonas fluorescens Lp1. Material Research Bulletin 2013; 48:1830-1836.
- Janusz G, Kucharzyk KH, Pawlika A, Staszczaka M, Paszczynskic AJ. Fungal laccase, manganese peroxidase and lignin peroxidase: Gene expression and regulation. Enzyme Microbial Technology 2013; 52: 1-12.
- Passarini MRZ, Rodrigues MVN, DaSilva M, Sette LD. Marine derived filamentous fungi and their potential application for polsycyclic aromatic hydrocarbon bioremediation. Marine Pollution Bulletin 2011; 62: 364-370.
- Maijala P, Kleen M, Westin C, Poppius-Levlin K, Herranen K, Lehto JH, Reponen P, Mäentausta O, Mettala A, Hatakka A. Biomechanical pulping of softwood with enzymes and white-rot fungus Physisporinus rivulosus. Enzyme Microbial Technology 2008; 43: 169-177.
- Susla M, Novotny C, Erbanova P, Svobodova K. Implication of Dichomitus squalens manganese-dependent peroxidase in Dye decolorization and cooperation of the enzyme with laccase. Folia Microbiologica 2008; 53: 479-485.
- Sahadevan LDM, Misra CS, Thankamani V. Characterization of lignin-degrading enzymes (LDEs) from a dimorphic novel fungus and identification of products of enzymatic breakdown of lignin. 3 Biotech 2016; 6: 56.
- Indira Priyadarsini R, Bhuvaneswari V, Suresh Kumar K. Isolation, Identification and Phylogenetic Analysis of White Rot Fungus and Heterologous Expression of Gene Encoding Laccase. Journal of Applied Sciences in Environmental Sanitation 2011; 6: 69-83.
- Sette LD, Oliveira VM, de Rodrigues MF. Microbial lignocellulolytic enzymes: industrial applications and future perspectives. Microbiology Australia 2008; 29: 18-20.
- Viswanath B, Rajesh B, Janardhan A, Praveen Kumar A, Narasimha G. Fungal Laccases and Their Applications in Bioremediation. Enzyme Research 2014; 1-21.
- Marbach I, Harel E, Mayer AM. Pectin, a second inducer for laccase production by Botrytis cinerea. Phytochemistry 1985; 24(11): 2559-2561.
- Reddy CA, Mathew Z. "Bioremediation potential of white rot fungi," in Fungi in Bioremediation, G M Gadd, Ed, Cambridge University Press, Cambridge, UK 2001.
- Hublik G, Schinner F. Characterization and immobilization of the laccase from Pleurotus ostreatus and its use for the continuous limination of phenolic pollutant. Enzyme Microbial Technology 2000; 27: 330-336.
- Arun A, Raja PP, Arthi R, Ananthi M, Kumar KS, Eyini M. Polycyclic aromatic hydrocarbons(PAHs) biodegradation by basidiomycetes fungi, Pseudomonas isolate, and their co cultures: comparative in vivo and in silico approach. Applied Biochemistry and Biotechnology 2008; 151: 132-142.
- Robinson T, Chandran B, Nigam P. Studies on the production of enzymes by white-rot fungi for the decolourisation of textile dyes. Enzyme Microbial Technology 2001; 2: 575-579.
- Asgher M, Irshad M, Iqbal HMN. Purification and characterization of lignin peroxidase produced by Schizophyllum commune IBL-06 using banana stalk in solid state cultures. Bio Resources 2012a; 7: 4012-4021.
- Zeng G, Zhao H, Huang D, Lai C, Huang C, Wei Z, Xu P, Li N, Zhang C, Li F, Cheng M. Purification and biochemical characterization of two extracellular peroxidases from Phanerochaete chrysosporium responsible for lignin biodegradation. International Biodeterioration and Biodegradation 2013; 85: 166-172.
- Junnarkar N, Pandhi N, Raiyani N, Bhatt N, Raiyani R. Production of LiP by Phanerochaete chrysosporium MTCC 787 Through Solid State Fermentation of Wheat Straw and Assessing its Activity Against Reactive Black B. International Journal of Advanced Research 2016; 4(1): 812-819.
- Asgher M, Iqbal HMN, Asad MJ. Characterization of purified and xerogel immobilized novel lignin peroxidase produced from Trametes versicolor IBL-04 using solid state medium of corncobs. BMC Biotechnology 2012b; 12: 46.
- Knezevic A, Milovanovic I, Stajic M, Vakojevic J. Trametes suaveolens as ligninolytic enzyme producer, Journal of Natural Sciences, 124 (2013) 437-444.
- Silva MLC, Souza VB, Santos VS, Kamida HM, Vasconcellos-Neto JRT, Góes-Neto A, Koblitz MGB. Production of manganese peroxidase by Trametes villosa on unexpensive substrate and its application in the removal of lignin from agricultural wastes. Advances in Bioscience and Biotechnology 2014; 5: 1067-1077.
- Hakala TK, Hilden K, Maijala P, Olsson C, Hatakka A. Differential regulation of manganese peroxidases and characterization of two variable MnP encoding genes in the white-rot fungus Physisporinus rivulosus. Applied Microbiology and Biotechnology 2006; 73: 839-49.
- Hilden K, Martinez AT, Hatakka A, Lundell T.The two manganese peroxidases Pr-MnP2 and Pr-MnP3 of Phlebia radiata, a lignin-degrading basidiomycete, are phylogenetically and structurally divergent. Fungal Genetics and Biology 2005; 42: 403-19.
- Asgher M, Iqbal HMN, Asad MJ. Kinetic characterization of purified laccase produced from Trametes versicolor IBL-04 in solid state bioprocessing of corncobs. Bio Resources 2012c; 7: 171-1188.
- Iqbal HMN, Ahmed I, Zia MA, Irfan M. Purification and characterization of the kinetic parameters of cellulase produced from wheat straw by Trichoderma viride under SSF and its detergent compatibility. Advances in Bioscience and Biotechnology 2011; 2: 49-156.
- Jayani RS, Saxena S, Gupta R. Microbial pectinolytic enzymes: A review, Process Biochemistry 2005; 40: 2931-2944. ± ± ± ± ± ±










 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License