IJCRR - 5(7), April, 2013
Pages: 78-83
Date of Publication: 18-Apr-2013
Print Article
Download XML Download PDF
CUTANEOUS ADVERSE DRUG REACTION MONITORING OF DIFFERENT DRUGS IN DERMATOLOGY OPD OF A TERTIARY CARE TEACHING HOSPITAL
Author: Monalisa Jena, Swati Mishra, M. Panda, S.S. Mishra
Category: Healthcare
Abstract:Background: Drugs can cure, suppress or prevent a disease and are usually beneficial to humans. However, they can also produce undesirable/harmful effects, which are known as adverse drug reactions. These are important cause of morbidity, hospitalization, increased health expenditure and even death. Cutaneous adverse drug reactions are among the most frequent adverse drug reactions. Active search is essential for identification of these, as patients may tend to downplay the causal association between drug use and the subsequent cutaneous manifestation. Objective: To observe the types of drug induced cutaneous drug reactions in the patients attending to out patients department of Dermatology in a tertiary care teaching hospital, Bhubaneswar, Odisha and find out the incidence, causal relationship with final outcome ofcutaneous drug reactions. Patients and Methods: A prospective study involving 100 patients attending to the Dermatology Outpatient department was observed during the period of six months to find the patients with CADRs using self-reporting method for selection of cases in the adverse drug reaction monitoring form by CDSCO, India. Causality was assessed using WHO-UMC Causality assessment Scale. Results were analyzed using suitable statistical methods. Results and conclusion: Cutaneous reactions are the most common manifestations of adverse drug reactions. The pattern of adverse drug reactions and the drugs causing them is remarkably different in our population. Knowledge of these drug eruptions, the causative drugs and the prognostic indicators is essential for clinicians for diagnosis and prevention of adverse drug reactions. It is recommended to advise patients to carry a card or an emergency identification of offending drugs in their wallets that list the drug allergies and/or intolerances.
Keywords: Adverse drug reaction, cutaneous drug eruption, CDSCO
Full Text:
INTRODUCTION
Drugs are always related with risk of adverse reactions, no matter how safe and efficacious they are. Adverse drug reaction is a response to a drug that is noxious and occurs at doses normally used in man for the prophylaxis, diagnosis or therapy of disease, or for modification of physiological function [1]. It is an unexpected, undesired, and unintended or a toxic consequence of drug administration. Cutaneous drug eruptions are most common types of adverse reaction to drug therapy, with an overall incidence rate of 2%–3% in hospitalized patients [2]. Any medicine can induce skin reactions, and certain drug classes, such as non-steroidal anti-inflammatory drugs, antibiotics and antiepileptics, have drug eruption rates approaching 1%–5% [2]. It was seen that most drug eruptions are serious, some are even severe life threatening. Serious reactions include angio-oedema, erythroderma, Stevens– Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN)[3] .The incidence of the these drug eruptions is directly proportional to the number of drugs prescribed. [4] The historytaking for drug intake is very important, which includes questioning direct, indirect and suggestive. It takes time, but answers are golden in case of cutaneous drug reactions and druginduced dermatitis. [5], [6], [7]Safe use of the drugs is the responsibility of health care professional and a proper knowledge of adverse cutaneous drug reaction related information may be helpful in prevention of it.
PATIENTS AND METHODS
A prospective study spread over 6 months duration, from May 2012 to October 2012 was carried out in the Dermatology OPD in collaboration with Dept. of Pharmacology, IMS and SUM Hospital, Bhubaneswar, Odisha for recording the Cutaneous adverse drug reactions. 100 patients were enrolled for this study using self-reporting method for selection of cases using ADR reporting form by CDSCO. Following patients were excluded:
(i) Patients not willing to take part in the study,
(ii) Patients dropping out the study at any stage at their will
(iii) Patients lost to follow up.
Inclusion criteria’s:
(i) Patients of all age groups and either sex
(ii) Developing a suspected adverse cutaneous drug reactions following use of any medication were included in the study.
Detailed clinical history was taken in a predesigned proforma. History of drug ingestion, self-administration and H/O symptoms, other previous skin and systemic diseases, or any other illness was taken. Thorough clinical examination was carried out. Skin, hair, nail and mucosa (eye, oral and genital) were examined. Diagnosis was confirmed by Dechallenge test (disappearance of signs and symptoms after discontinuation of offending drugs) .Data of suspected cutaneous adverse drug reactions was entered in CDSCO adverse drug reactions reporting form, India. Patients’ consent was obtained to take photos. WHO definition and classifications of adverse drug reactions were followed. The initial history included a recording of all prescriptions and nonprescription drugs taken within the last one month, including dates of administration and dosage and also the history of previous drug exposure and reactions, family history of drug reactions, features and severity of adverse drug reactions etc. Approval from institutional ethics committee was taken before starting the study. Consent from patient was also taken. Causality assessment was done using WHO-Uppsala monitoring center scale, 2002. Cases with a certain, probable or possible were recorded. Relevant laboratory investigations were undertaken to arrive at a clinical diagnosis. Dechallenge was done. Rechallenge was not attempted. The data was compiled and subjected to descriptive statistical analysis.
RESULTS
100 patients among which 72 males and 28 females were included in this study (table no 1). The time required to develop cutaneous lesions between 1- 45 days after intake of drug were considered. Sex distribution of drug eruption indicates patients belongs to the 41-50 years age group which is 32% of the study population which is maximum followed by 21-30years(28%) and 31-40 years (20%)of the study population(table no 1). The youngest patient of our study belongs to 1year old and the oldest was 80years old.
In pattern of drug eruption and offending drugs:
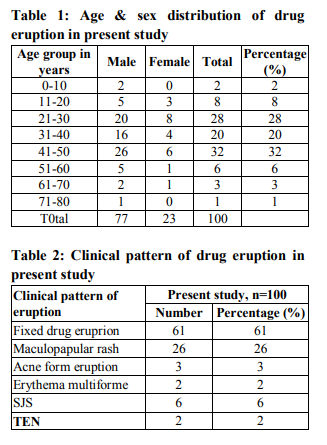
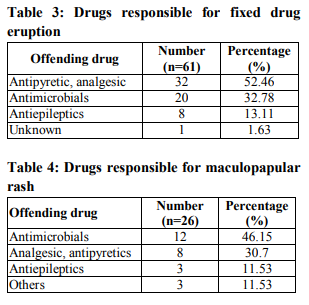
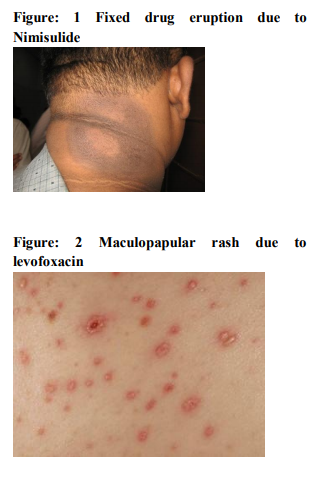
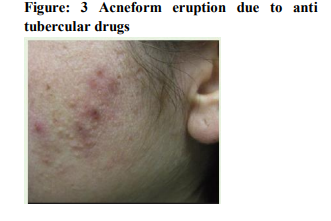
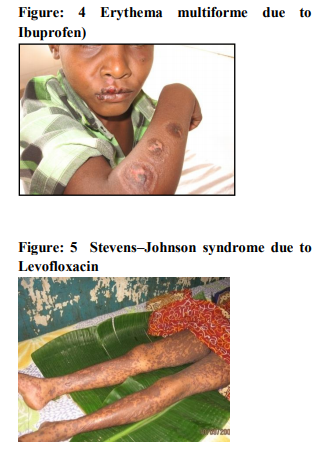
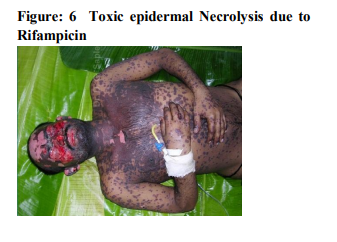
It was seen that cutaneous drug eruption which is commonest in our study was fixed drug eruption which was 61% of study population followed by maculopapular rashes 26%(table no:2). Fixed drug eruption most commonly occurs due to non steroidal anti inflammatory drugs (NSAIDs) around 52% of all fixed drug eruption patients followed by antimicrobials 32%(table no:3). Other drugs like antiepileptics are also responsible for the fixed drug eruption i.e only 13.11%.one case of fixed drug eruption was found due to unknown drug in our study population. The most common NSAIDs to produce fixed drug eruption is Nimisulide (figure no:1) whereas common antimicrobials were Fluoroquinolones, Azithromycine and Cephalosporins. Maculopapular rashes were the 2nd most common cutaneous drug eruption for which the most important and common offending drug was antimicrobials (figure no: 2) followed by analgesics and antipyretics (table no: 4).Some other drugs like antiepileptics, antimalarials, antitubercular drugs were also responsible for development of maculopapular rashes. In our study 3 patients presented with acne form eruptions out of which 2 were due to antitubercular drugs(INH, Rifampicin, Pyrazinamide, Ethambutol) (figure no:3)and one was due to Ampicillin. 2 cases of erythema multiforme were seen because of Ibuprofen (figure no:4). There were 6 cases of Stevens–Johnson syndrome out of which Ibuprofen induced were three in number, two were due to antimicrobials ( Levofloxacin, Cefixime)( Table no :2,figure no:5), one was due to unknown drug. Amongst these two were severe which were managed in intensive care with positive result. There were two cases of toxic epidermal necrolysis, out of which one case was due to Rifampicin( Figure no:6) which was severe but responded well to immediate management. Another one was due to unknown drug which was proved fatal.
DISCUSSION
Among 100 patients of our study population, 72 were male and 28 were female. So percentage of male patients was more affected by cutaneous drug eruptions than females in our study population. The ratio of male to female patients comes out as 2.57. It is similar as a study done in a North-Indian tertiary care center which reported male preponderance [8] .In some study reports female preponderance also has been found. One possibility to explain the gender difference may be due to their genetic makeup or adherence to the drug more due to variability in the number of the male and female patient attending in different center and so frequently attending patient has higher chances of adverse drug reactions. Next component of the study was to find out whether there is any association between different age groups of the patients and the incidence of cutaneous drug eruptions. In this study among various age group 41-50 years age group had preponderance but in some other Indian studies the young adults had the preponderance [9] . The commonest pattern was fixed drug eruption (61%), followed by maculopapular rashes (26%) and Stevens–Johnson syndrome (6%). According to Pudukadan D et al study, the pattern of cutaneous drug eruption which was commonest was fixed drug eruption (31.1%), followed by maculopapular rash (12.2%) which was similar to our study .[4] Malhotra et al study reported morbilliform rash (29.63%), and urticaria in( 9.26% )as common patterns of reaction. [10] Jhaj et al. reported morbilliform rashas commonest pattern followed by, urticaria (21%).[5]Most important reason for intake of the above drugs are pain, fever and infection. The commonest culprit of fixed drug eruption in our study were NSAIDs, differ from the study by Singh et al where cotrimoxazole was the commonest cause [6] NSAIDs and cotrimoxazole were the common cause of drug eruption in the study by Shrivastav et al. [7],[11] Quinolones were a common cause of maculopapular rash and photosensitivity in our study which indicates increased use of quinolones. [12] Ibuprofen was the commonest cause of erythema multiforme (EM) and Stevens Johnson's syndrome (SJS) in our study. From the report of Halevi et al Stevens Johnson's syndrome is due to acetaminophen, [12] while in the study by Devik et al carbamazepine was the commonest offending drug. [13] .The incidence of isoniazide induced acneiform eruptions (0.53%) described in a study by Sharma PP,[14] while we had 3 cases of acne form eruptions due to isoniazide. A high incidence of toxic epidermal necrolysis and Stevens Johnson's syndrome has also been reported from a North-Indian hospital,[15] while western studies have shown very low incidence[16]
Our study had some inherent limitations like: Small sample size, confined to the outpatient department (OPD) of the skin and VD department only and a short period of six months and unable to do the rechallenge. Yet the study clearly provides the baseline data for comparing with other similar studies at the level of state, country and the world. It also provided the information regarding the management of the cutaneous adverse drug reactions and their outcome thus making the drug therapy safer and more rational. This study has been a further step in the direction of strengthening the activity of pharmacovigilance in this part of the country.
CONCLUSION
It’s the responsibility of clinicians and clinical pharmacists to recognize clinically important ADRs and report them to strengthen the pharmacovigilance activity. This was a prospective and observational study for detection of CADRS and analyzing various facets of the same. The study has revealed many interesting points and has given us insight to carry out further studies of similar type in future so as to derive better information. It is concluded from the above study that by knowing the incidence, morphological patterns and causative agents of various adverse cutaneous drug reactions, many common and serious adverse affects due to drugs can be avoided. Due to lack of interest in ADR monitoring and poor response of the clinician for pharmacovigilance many of them go unreported. It is our contention that the use of high risk drug should be carefully monitored for ADRs and awareness should be created in patients by treating physician so that the morbidity and mortality by the use of the drug should be decreased.
ACKNOWLEDGEMENT
Authors acknowledge the great help received from the scholars whose articles cited and included in references of this manuscript. The authors are also grateful to authors / editors / publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed. Authors are grateful to IJCRR editorial board members and IJCRR team of reviewers who have helped to bring quality to this manuscript.
References:
1. WHO. International drug monitoring: the role of natTioenchal centres. Rep Ser WHO 1972, no 498
2. Lauraence DR, Bennett PN, Brown MJ. Unwanted effects and adverse drug reactions.Clinical Pharmacology. 8th edn. Churchill Livingstone 1997: 121-137.
3. Dennis L, Kasper MD, Eugene Burunwald MD, Anthony S. Fauci MD, Stephen L Hauser MD, Dan L Longo MD, Larry Jameson J. MD. Ph.D. Harrison’s Principles of internal medicine, 16th edition, Vol – 1, Medical publishing Division 2005: 318-320.
4. Pudukadan D, DM Thappa. Adverse cutaneous drug reactions: Clinical pattern and causative agents in a tertiary care centre in South India. Indian J Dermatol Venereol Leprol 2004; 70:20-4.
5. Jhaj R, Malhotra S, Bhargava VK, Uppal R. Cutaneous adverse reactions in in-patients in a tertiary care hospital. Indian J Dermatol Venereol Leprol ,1999; 65:14-7.
6. Singh KK, Krupashankar DS , Shrinivas CR, Naik RPC. Study of 33 cases of fixed drug eruption, Indian J Dermatol Venereol Leprol ,1990; 56:123.
7. Shrivastava D, Singh SK, Kumar A. Adverse drug reaction monitoring in patients attending skin O.P.D at a teaching hospital. Indian J Pharmacol 2004; 36:S42.
8. Sharma VK, Sethuram G, Kumar B. Cutaneous adverse drug reactions: Clinical pattern and causative agents - A 6 year series from Chandigarh, India. J Postgrad Med 2001; 47:95-99.
9. Barbara S.M, In: Shargel, L., Eds., Comprehensive Pharmacy Review, 4th Edn., Lippincott Williams and Wilkins, London, 2001, 416.
10. Malhotra S, Dogra A, Chopra SC, Gupta C. Cutaneous adverse drug reactions-one year pharmacovigilance study in a tertiary care hospital. Indian J Pharmacol, 2004; 36:S41-2.
11. Prasad PV. A study of Dapsone syndrome at a rural teaching hospital in south India. Indian J Dermatol Venereol Leprol, 2001; 67:69-71.
12. Halevi A, Ben-Amitai D, Garty BZ. Toxic epidermal necrolysis associated with acetaminophen ingestion. Ann Pharmacother 2000; 34:32-4.
13. Devi K, George Sandhya, Criton S, Suja V. Carbamazepine-The commonest cause of toxic epidermal necrolysis and Steven Johnson syndrome: A study of 7 years. Indian J. Dermatol Venereol Leprol 2005; 71:325-8.
14. Sharma RP, Kothari AK, Sharma NK. Antitubercular drugs and acne form eruptions :Indian J Dermatol Venereol Leprol, 1995; 61:26-7.
15. Uppal R, Jhaj R, Malhotra S. Adverse drug reactions among inpatients in a North Indian referral hospital. Natl Med J India 2000; 13:16-18.
16. Naldi L, Conforti A, Troncon MG, Venegoni M ,Caputi A, Ghiotto E, et al.. Cutaneous reactions to drugs. An analysis of spontaneous reports in four Italian regions. Brazilian J Clin Pharmacol 1999;48:839-846.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License